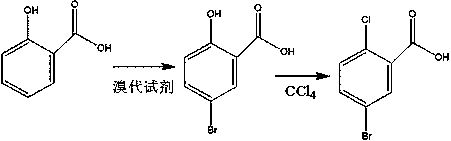
Preparation method of high-selectivity 5-bromo-2-chlorobenzoic acid
A kind of technology of chlorobenzoic acid and monobromine, applied in the field of preparation of 5-bromo-2-chlorobenzoic acid, can solve problems such as unsuitable for industrialized production, affecting production application, increasing production cost, etc. little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
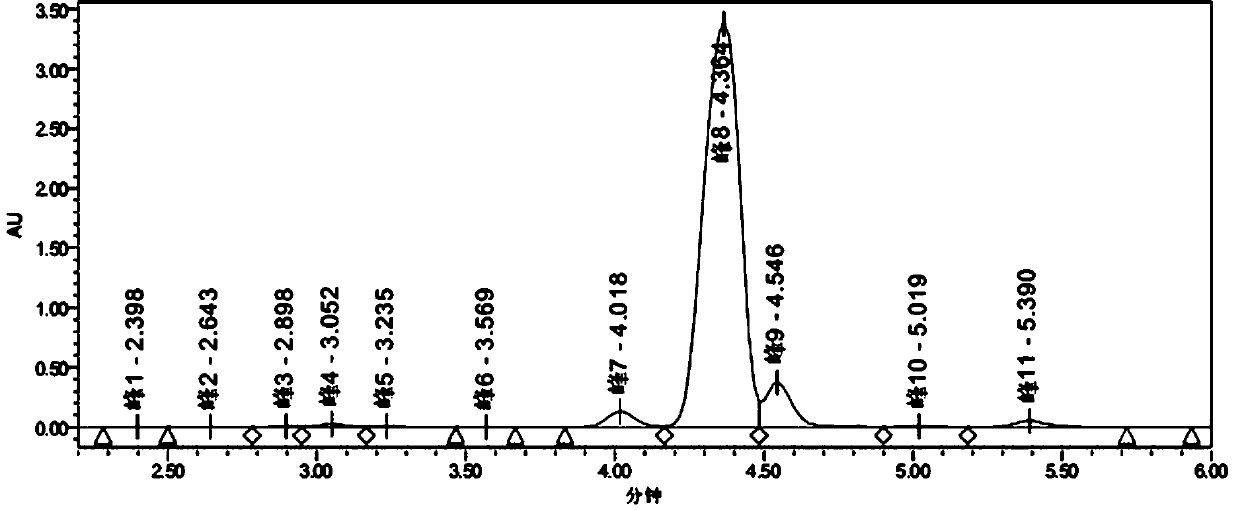
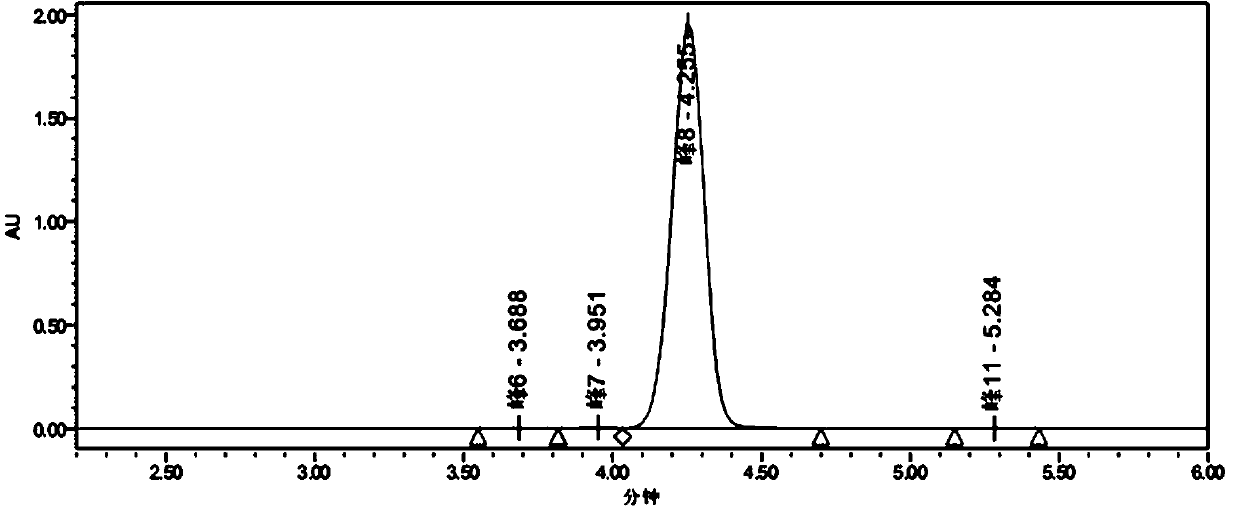
[0026] Add 4.7g (0.03mol) of 2-chlorobenzoic acid (0.03mol), concentrated sulfuric acid (40mL), and 0.936g (0.012mol) of sodium sulfide to a 250mL four-necked flask in sequence, stir at 30°C for 20 minutes until the solution is clear, then add 5.334g N-bromosuccinimide (0.03mol), continue to react at 30°C for 10 minutes, then slowly pour the solution into 80mL ice-water bath for crystallization to obtain crude 5-bromo-2-chlorobenzoic acid. The filter cake was put into a 250mL four-neck flask, 24mL of methanol and 36mL of water were added, the temperature was raised to 60°C, cooled naturally, and stirred for crystallization. Filter, wash with 20 mL of 40% methanol aqueous solution, and dry at 55°C for 6 hours to obtain 6.001 g of white solid, yield: 85.0%, HPLC purity: 99.6%. The liquid chromatogram of the primary crystallization product is as follows: figure 2 Shown:
[0027] sample catalyst Crude 5-bromo-2-chlorobenzoic acid content Impurity 4-bromo-2-chlor...
Embodiment 2
[0029] Into a 250mL four-neck flask, add 4.7g (0.03mol) of 2-chlorobenzoic acid (0.03mol), concentrated sulfuric acid (40mL), and 0.662g (0.006mol) of potassium sulfide in sequence, stir at 40°C for 20 minutes until the solution is clear, and then add N- Bromosuccinimide 4.271g (0.024mol), continue to react at 40°C for 60 minutes, then slowly pour the solution into 80mL ice-water bath for crystallization to obtain crude 5-bromo-2-chlorobenzoic acid. Add the filter cake to a 250mL four-neck flask, add 24mL of acetic acid and 36mL of water, heat up to 60°C, cool naturally, and stir to crystallize. Filter, wash with 20 mL of 40% acetic acid aqueous solution, and dry at 55°C for 6 hours to obtain 5.973 g of white solid, yield: 84.6%, HPLC purity: 99.7%.
Embodiment 3
[0031] Add 4.700g (0.03mol) of 2-chlorobenzoic acid (0.03mol), concentrated sulfuric acid (40mL), and 2.269g (0.018mol) of sodium sulfite to a 250mL four-necked flask in sequence, stir at 10°C for 20 minutes until the solution is clear, then add N-bromo 3.204 g (0.018 mol) of substituted succinimide was reacted at 10°C for 120 minutes, and then the solution was slowly poured into an 80 mL ice-water bath for crystallization to obtain crude 5-bromo-2-chlorobenzoic acid. Filter, add the filter cake into a 250mL four-neck flask, add 60mL of ethanol, heat up to 60°C, cool naturally, and stir to crystallize. Wash with 20 mL of ethanol solution and dry at 55°C for 6 hours to obtain 5.958 g of white solid, yield: 84.3%, HPLC purity 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com