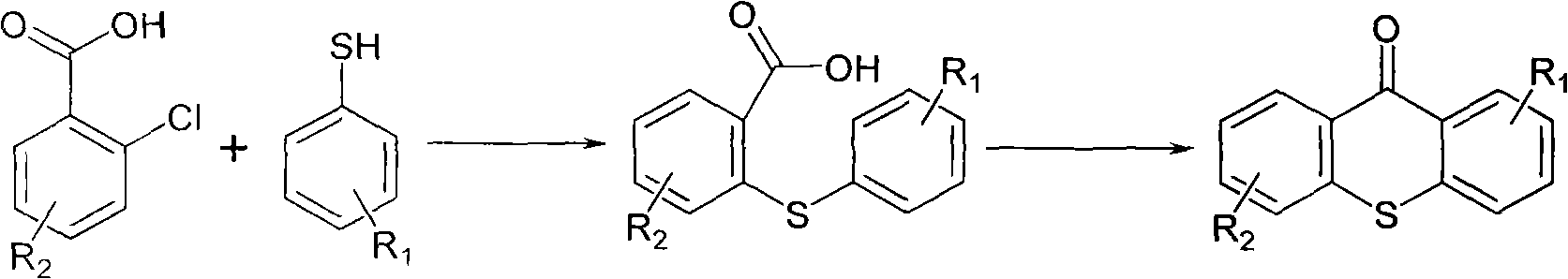
Preparation method of 2-isopropyl thioxanthone and derivatives thereof
A technology of isopropylthioxanthone and derivatives is applied in the field of preparation of 2-isopropylthioxanthone and derivatives thereof, and can solve the problem that the acylation raw material 4-isopropylanisole is expensive, The tetralin solvent is expensive and complicated to operate, so as to achieve the effect of low price, low cost and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Preparation of 2-isopropylthioxanthone
[0020] Mix 130g (0.83mol) o-chlorobenzoic acid, 120g (0.79mol) 4-isopropylthiophenol, 68g (1.70mol) sodium hydroxide, 50mL water, heat to 80°C and stir for 0.5 hours, add 520mL toluene , Heat reflux for dehydration. The resulting solution is protected with nitrogen, sealed and pressurized at 1MPa, heated to 210°C, reacted for 10 hours, cooled down to 90°C, adjusted to pH 2 with 33% industrial hydrochloric acid, separated the water layer, maintained the temperature, and the organic phase Wash with water until neutral.
[0021] Add 300mL 98% concentrated sulfuric acid to the organic phase, stir, heat the reaction for 10h, add water, stir for 0.5h, stand still, divide the water phase, wash the organic phase with 500mL water to neutrality, decompose under reduced pressure, and use 150mL absolute ethanol Recrystallized to obtain 160 g of light yellow solid, namely: 2-isopropylthioxanthone with a content of 99.5%.
[0022] Melti...
Embodiment 2
[0024] Example 2: Preparation of 2-isopropylthioxanthone
[0025] Mix 130g (0.83mol) o-chlorobenzoic acid, 120g (0.79mol) 4-isopropylthiophenol, 68g (1.70mol) sodium hydroxide, 50mL of water, heat to 80℃ and stir for 0.5 hours, add mineral spirits 520mL, heated to reflux for dehydration, the resulting solution was protected with nitrogen, sealed and pressurized at 1MPa, heated to 200°C, and reacted for 12 hours. Cool down to 80°C, adjust the pH of the solution to 3 with 33% industrial hydrochloric acid, separate the water layer, maintain the temperature, and wash the organic phase with water to neutrality.
[0026] Add 300mL 98% concentrated sulfuric acid to the organic phase, stir, heat the reaction for 8h, add water, stir for 0.5h, stand still, divide the water phase, wash the organic phase with 500mL water to neutrality, de-solve under reduced pressure, use 150mL absolute ethanol Recrystallized to obtain 164g of light yellow solid, namely: 2-isopropylthioxanthone with a content...
Embodiment 3
[0027] Example 3: Preparation of 2-isopropylthioxanthone
[0028] Mix 130g (0.83mol) o-chlorobenzoic acid, 120g (0.79mol) 4-isopropylthiophenol, 68g (1.70mol) sodium hydroxide, 50mL water, heat to 80℃ and stir for 1 hour, add 520mL diesel oil , Heated to reflux for dehydration, the solution obtained for 1 hour was protected with nitrogen, sealed and pressurized at 1MPa, heated to 190°C, and reacted for 11 hours. Cool down to 90°C, adjust the pH of the solution to 2 with 33% industrial hydrochloric acid, separate the water layer, maintain the temperature, and wash the organic phase with water to neutrality.
[0029] Add 300mL 98% concentrated sulfuric acid to the organic phase, stir, heat the reaction for 10h, add water, stir for 0.5h, stand still, divide the water phase, wash the organic phase with 500mL water to neutrality, decompose under reduced pressure, and use 150mL absolute ethanol Recrystallized to obtain 157 g of light yellow solid, namely: 2-isopropylthioxanthone with a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com