Novel method for synthesizing dapagliflozin intermediate compound
A new method and technology for intermediates, applied in the field of synthesizing dapagliflozin intermediates, can solve the problems of inconvenient transportation of oxalyl chloride, incomplete reaction of raw materials, troublesome tetrahydrofuran, etc., and achieve the effects of easy recovery of tetrahydrofuran, clean reaction and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
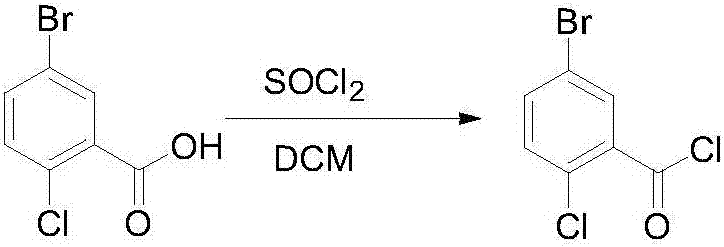
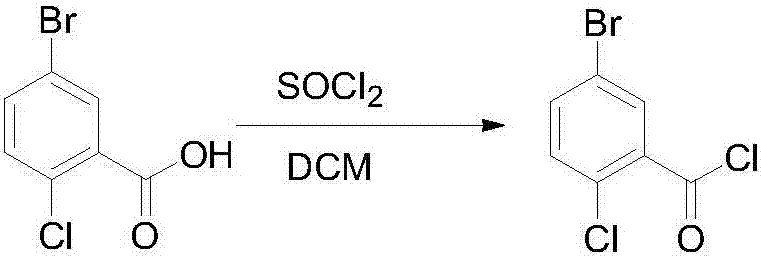
[0031] Step 1. Dissolve 5-bromo-2-chlorobenzoic acid (100g, 0.43mol) in 500ml of dichloromethane, add 1g of pyridine for catalysis, add thionyl chloride (60.5g, 0.51mol) dropwise at room temperature, and heat up to Reflux at 40°C for 3.5 hours, use TLC to detect that the reaction is complete, and concentrate the dichloromethane under reduced pressure to obtain 104 g of crude 5-bromo-2-chlorobenzoyl chloride (yield 97%), which is directly used in the next step without further purification;
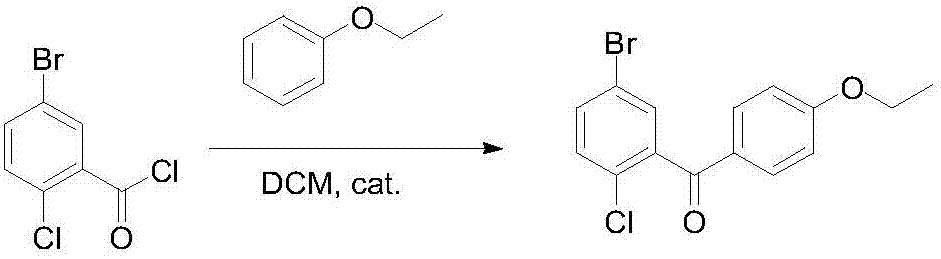
[0032] Step 2: Using 500ml of dichloromethane as a solvent, add 10g of solid acid catalyst (preparation method of solid acid attached below) into the reaction kettle, add phenetole (50g, 0.41mol) at room temperature, stir for 2h after the dropwise addition, and then dropwise add The concentrated solution of 5-bromo-2-chlorobenzoyl chloride obtained in step 1 was reacted at a temperature of 30°C for 3-4 hours. After the reaction was completed, it was filtered and concentrated to obtain 5-brom...
Embodiment 2
[0035] Step 1, 5-bromo-2-chlorobenzoic acid (100g, 0.43mol) was dissolved in 500ml of dichloromethane, catalyzed by adding 1g of pyridine, and thionyl chloride (76.1g, 0.645mol) was added dropwise at room temperature, and the temperature was raised to Reflux at 40°C for 3 hours, use TLC to detect that the reaction is complete, and concentrate the dichloromethane under reduced pressure to obtain 102.5 g of crude 5-bromo-2-chlorobenzoyl chloride (yield 95%), which is directly used in the next step without further purification ;
[0036] Step 2. Using 500ml of dichloromethane as a solvent, add 10g of solid acid catalyst (preparation method of solid acid attached below) into the reaction kettle, add phenetole (59.6g, 0.49mol) at room temperature, stir for 2h after the addition, and then drop Add the concentrated solution of 5-bromo-2-chlorobenzoyl chloride obtained in step 1, and react at a temperature of 30°C for 4 hours. After the reaction is completed, filter and concentrate to...
Embodiment 3
[0039]Step 1. Dissolve 5-bromo-2-chlorobenzoic acid (100g, 0.43mol) in 500ml of dichloromethane, add 1g of pyridine to catalyze, add thionyl chloride (101.5g, 0.86mol) dropwise at room temperature, and heat up to Reflux at 40°C for 5 hours, use TLC to detect that the reaction is complete, and concentrate the dichloromethane under reduced pressure to obtain 103 g of crude 5-bromo-2-chlorobenzoyl chloride (yield 96%), which is directly used in the next step without further purification;
[0040] Step 2: Using 500ml of dichloromethane as a solvent, add 10g of solid acid catalyst (preparation method of solid acid attached below) into the reaction kettle, add phenetole (50g, 0.41mol) at room temperature, stir for 1h after the dropwise addition, and then dropwise add The 5-bromo-2-chlorobenzoyl chloride concentrate obtained in step 1 was reacted at a temperature of 35°C for 5 hours. After the reaction was completed, it was filtered and concentrated to obtain 5-bromo-2-chloro-4-ethoxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com