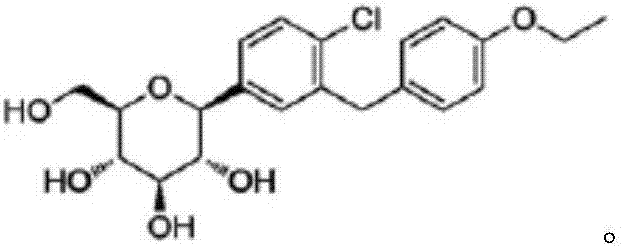
Preparation method of Dapagliflozin intermediate used for treating II-type diabetes
An intermediate, the technology of chlorobenzoyl chloride, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of many by-products and difficulty in purification, and achieve the effects of short reaction time, mild conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
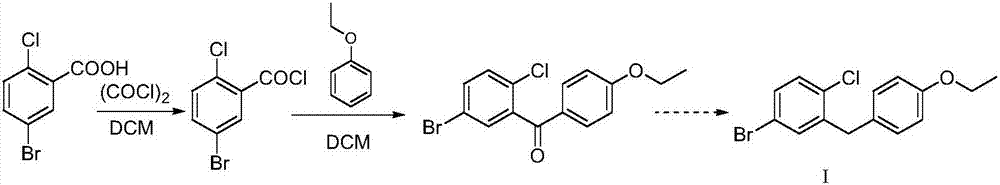
[0027] A method for preparing a dapagliflozin intermediate for treating type Ⅱ diabetes, comprising the following steps:
[0028] 1) Under nitrogen protection, 235.5g (1mol) of 5-bromo-2-chlorobenzoic acid, 380.9g (3mol) of oxalyl chloride and a catalytic amount of DMF (2ml) were added to 1500ml of anhydrous dichloromethane at room temperature for contact reaction 1 ~2 hours, remove the solvent and unreacted oxalyl chloride under reduced pressure to obtain 5-bromo-2-chlorobenzoyl chloride;
[0029] 2) Under nitrogen protection, mix 25 g of tert-butyldimethylsilyl chloride and 5-bromo-2-chlorobenzoyl chloride obtained in step 1) in anhydrous dichloromethane for 30 minutes at 0 to 10°C, and then keep Add 134.4 g (1.1 mol) of phenetole and 194.6 g (1.2 mol) of ferric chloride to the reaction system in sequence, stir for 3 hours, and when the reaction is complete, pour into ice water to quench the reaction, extract with dichloromethane, wash with water , and concentrated to obtai...
Embodiment 2
[0033] A method for preparing a dapagliflozin intermediate for treating type Ⅱ diabetes, comprising the following steps:
[0034] 1) Under nitrogen protection, 23.6g (0.1mol) of 5-bromo-2-chlorobenzoic acid, 25.4g (0.2mol) of oxalyl chloride and a catalytic amount of DMF (0.2ml) were added to anhydrous dichloromethane and contacted at room temperature React for 1 to 2 hours, remove the solvent and unreacted oxalyl chloride under reduced pressure to obtain 5-bromo-2-chlorobenzoyl chloride;
[0035] 2) Under nitrogen protection, mix 2.1 g of tert-butyldimethylsilyl chloride and 5-bromo-2-chlorobenzoyl chloride obtained in step 1) in anhydrous dichloromethane for 30 minutes at 0 to 10°C, and then Keeping the temperature, 14.7g (1.2mol) of phenetole and 17.8g (1.1mol) of ferric chloride were successively added to the reaction system, and the reaction was stirred for 5 hours. After the reaction was completed, it was poured into ice water to quench the reaction, extracted with dichl...
Embodiment 3
[0037] A method for preparing a dapagliflozin intermediate for treating type Ⅱ diabetes, comprising the following steps:
[0038] 1) Under nitrogen protection, 23.6g (0.1mol) of 5-bromo-2-chlorobenzoic acid, 50.8g (0.4mol) of oxalyl chloride and a catalytic amount of DMF (0.3ml) were added to anhydrous dichloromethane and contacted at room temperature React for 1 to 2 hours, remove the solvent and unreacted oxalyl chloride under reduced pressure to obtain 5-bromo-2-chlorobenzoyl chloride;
[0039]2) Under nitrogen protection, mix 2.5 g of tert-butyldimethylsilyl chloride and 5-bromo-2-chlorobenzoyl chloride obtained in step 1) in anhydrous dichloromethane for 20 minutes at 0 to 10°C, and then Keeping the temperature, 14.7 g (1.2 mol) of phenetole and 19.5 g (1.2 mol) of ferric chloride were added to the reaction system in turn, and the reaction was stirred for 5 hours. After the reaction was completed, it was poured into ice water to quench the reaction, extracted with dichlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com