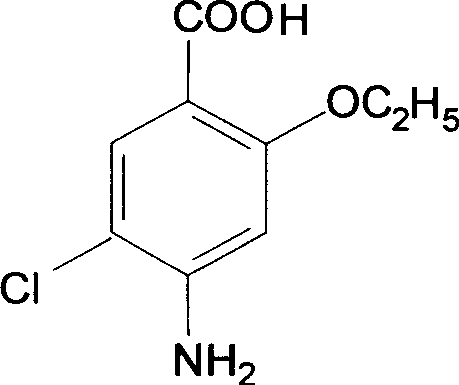
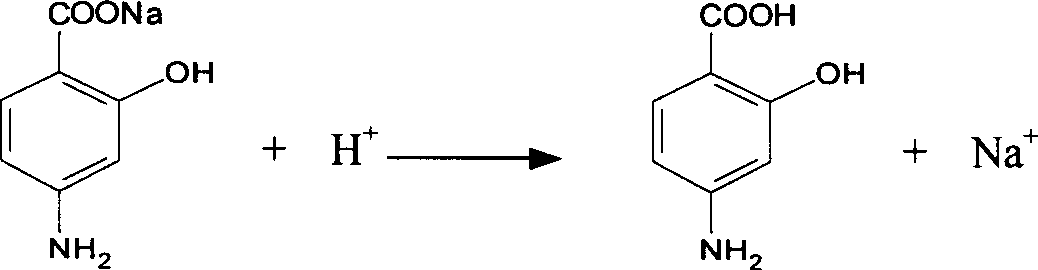
Synthesis of Important intermediate for mosapride citrate
A technology for mosapride citrate and its intermediates, applied in the field of medicine, can solve the problems affecting the popularization and application of mosapride citrate, difficult to meet the requirements of detection, and high price of iodoethane, so as to improve the synthesis Yield and finished product quality, stable response, and effect on alleviating price issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Preparation of the intermediate o-hydroxyp-aminobenzoic acid: 100 g of sodium o-hydroxyp-aminobenzoate dihydrate was dissolved in water, stirred, and concentrated hydrochloric acid was added dropwise to 150 g, cooled and filtered to obtain the intermediate o-hydroxyp-aminobenzoic acid, The yield was 96.5%.
[0017]
example 2
[0019] Preparation of intermediate o-hydroxy p-aminobenzoic acid methyl ester: intermediate o-hydroxy p-aminobenzoic acid was dissolved in methanol, stirred, concentrated sulfuric acid was added dropwise, refluxed, methanol was removed, and saturated K 2 CO 3 solution. Cool, stand still, and filter to obtain an off-white solid, which is methyl o-hydroxy p-aminobenzoate, with a yield of 79.7%.
[0020]
example 3
[0022] Preparation of the intermediate o-hydroxy p-acetamidobenzoic acid methyl ester: the intermediate o-hydroxy p-aminobenzoic acid methyl ester is dissolved in glacial acetic acid, stirred, acetic anhydride is added dropwise, and then the solvent is removed, NaOH solution is added to the raffinate, and the The white solid was filtered to obtain the intermediate methyl o-hydroxy p-aminobenzoate with a yield of 80.0%.
[0023]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com