Mefenamic acid short-process synthesis preparation and refining method
A technology of mefenamic acid and purification method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of cyanide reactions, etc., can solve the problems of high production cost, low product productivity, complicated technological process, etc., and achieves improved product quality. Yield, reduced production cost, simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
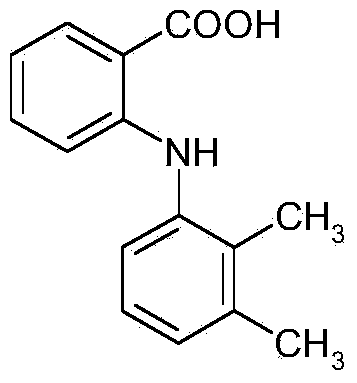
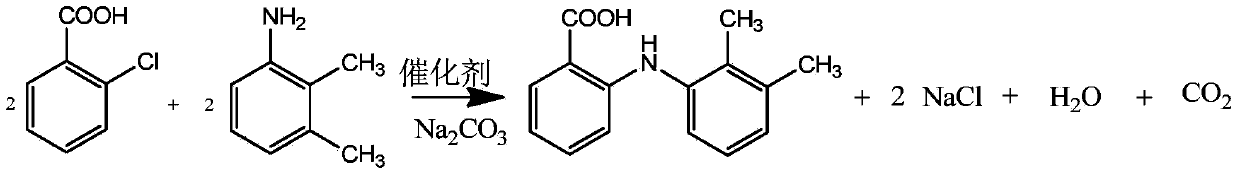
[0020] Embodiment 1, the synthetic preparation and refining method of mefenamic acid: (1) add 10kg N,N-dimethylformamide (DMF) in the condensation kettle, then drop into 4kg of o-chlorobenzoic acid and 2kg of salt of wormwood, Stir for 10±2 minutes; (2) Add 7kg of 2,3-dimethylaniline, stir evenly, and add 0.15kg of catalyst. Heat the temperature in the kettle to 120~128°C, react for 2 hours under constant stirring, add 3N hydrochloric acid solution dropwise into the kettle, and adjust the pH of the reaction solution to 4.3±0.2. Turn on the frozen brine to cool down, lower the internal temperature to 5±5°C, stir and crystallize for 30±2 minutes.
[0021] The crude product of mefenamic acid was obtained by filtration. (making the product is the sodium salt of mefenamic acid, so add hydrochloric acid to adjust the ph, and separate out the product (3). Add 10kg of N,N-dimethylformamide in the reaction vessel, then drop into 5kg of mefenamic acid crude product, Heat and stir to d...
Embodiment 2
[0022] Embodiment 2 is basically the same as Example 1, but wherein the molar ratio of each component is, o-chlorobenzoic acid: 2,3-dimethylaniline: sodium carbonate: DMF: catalyst=1: 1.5: 0.4: 4: 0.04 . The molar ratio of each component in step (3)-(6) is: crude mefenamic acid: medicinal charcoal: DMF=1:0.08:1.2.
Embodiment 3
[0023] Embodiment 3 is basically the same as Example 1, but wherein the molar ratio of each component is o-chlorobenzoic acid: 2,3-dimethylaniline: sodium carbonate: DMF: catalyst=1: 4: 1.2: 7: 0.18 . The molar ratio of each component in step (3)-(6) is: crude mefenamic acid: medicinal charcoal: DMF=1:0.25:3.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com