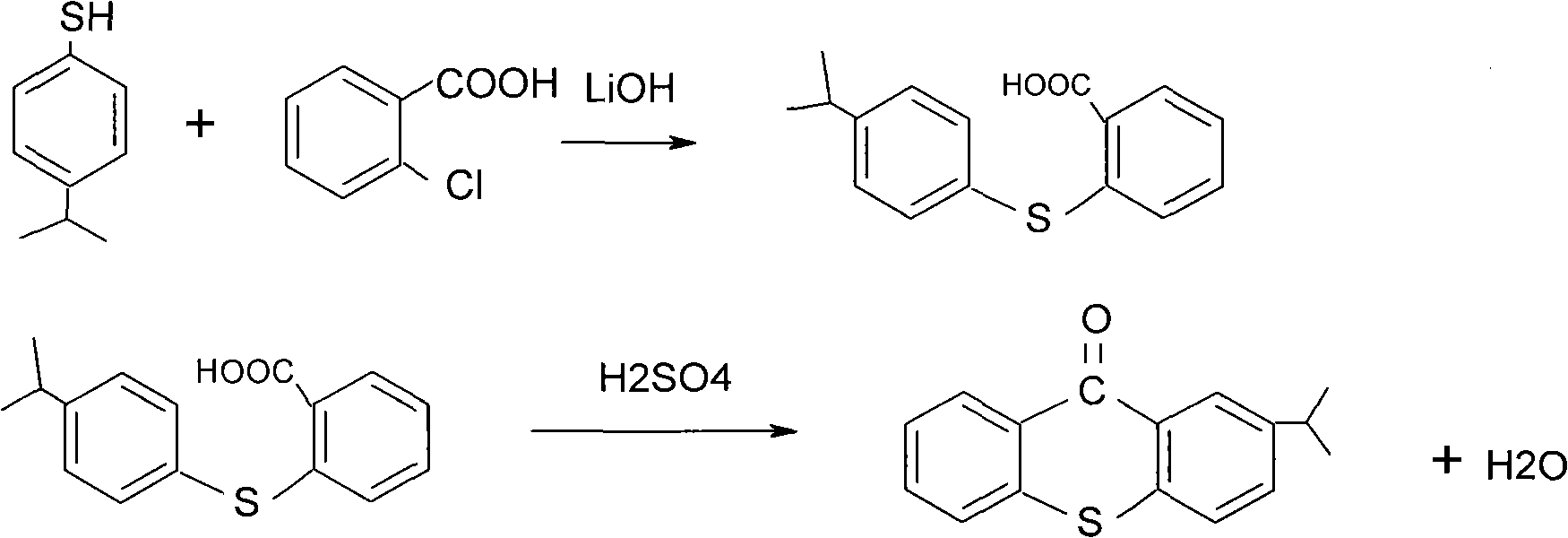
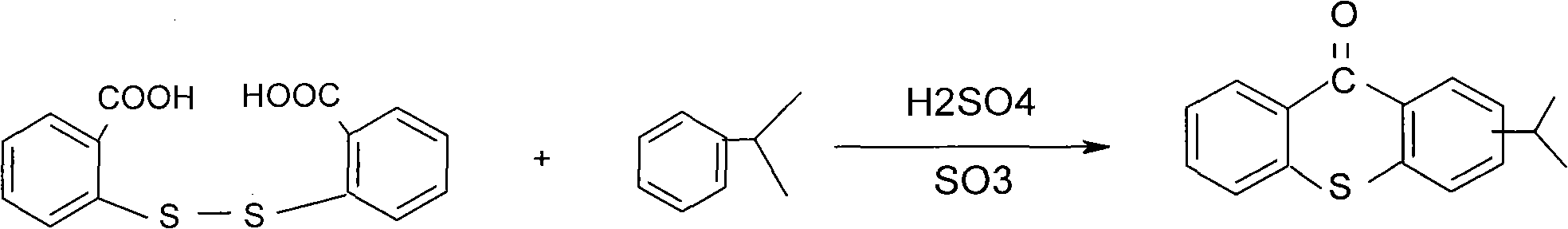
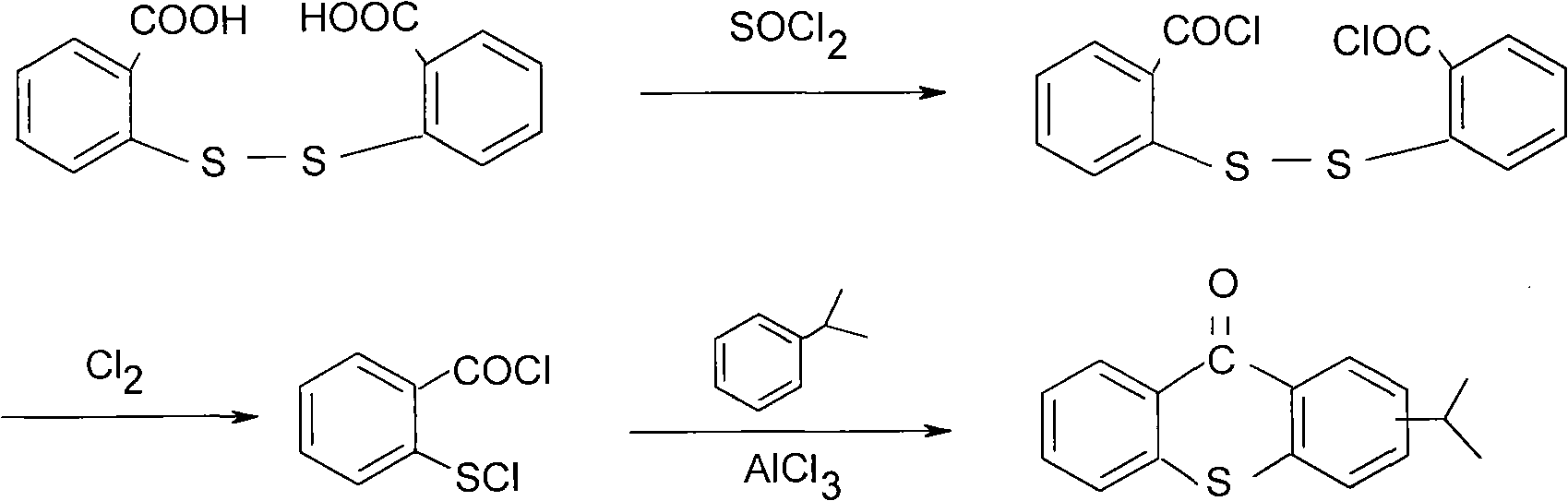
Preparation process of 2-isopropylthioxanthone
A technology of isopropylthioxanthone and p-isopropylthioxanthone is applied in the field of preparation of photoinitiator 2-isopropylthioxanthone, which can solve the problem of many synthesis process steps, high equipment requirements and many equipments. and other problems, to achieve the effect of high reaction yield, high product purity and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0013] Add 76 g of p-isopropyl thiophenol, 82 g of o-chlorobenzoic acid, 66 g of lithium hydroxide containing crystal water, and 150 ml of tetralin into a four-necked flask equipped with a reflux condenser. Separate the water, keep it at 180-190℃ for 6 hours, evaporate the tetralin under reduced pressure, then add 30% hydrochloric acid to neutralize and adjust the pH to 2-3, suction and filter, the filtrate recovers lithium chloride, and the filter cake is dried to obtain 133 Grams.
[0014] Add 270 ml of concentrated sulfuric acid to a dry three-necked flask, stir with ice water to cool to 10°C, and then add 133 g of the dried product in the previous step over 2 hours in batches. After adding and keeping warm for 2 hours, drain into water, add 150ml of toluene, let the layer separate, remove the acid water layer, add water to the oil layer and adjust the PH value to 7-8 with baking soda, divide the water layer, and use after the oil layer is stripped of toluene Recrystallization ...
example 2
[0016] Add 76 g of p-isopropylthiophenol, 75 g of o-chlorobenzoic acid, 66 g of lithium hydroxide containing crystal water, and 150 ml of tetralin into a four-necked flask equipped with a reflux condenser. Separate the water, keep it at 180-190℃ for 6 hours, evaporate the tetralin under reduced pressure, then add 30% hydrochloric acid to neutralize the pH to 2-3, filter with suction, recover the lithium chloride from the filtrate, and dry the filter cake to get 125 Grams.
[0017] Add 270 ml of concentrated sulfuric acid to a dry three-necked flask, stir with ice water to cool to 10°C, and then add 125 g of the dried product in the previous step over 2 hours in batches. After adding and keeping warm for 2 hours, drain into water, add 150ml of toluene, let the layer separate, remove the acid water layer, add water to the oil layer and adjust the PH value to 7-8 with baking soda, divide the water layer, and use after the oil layer is stripped of toluene Recrystallization from 150 ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com