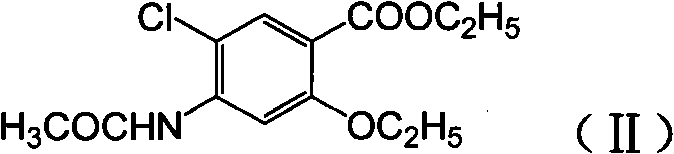
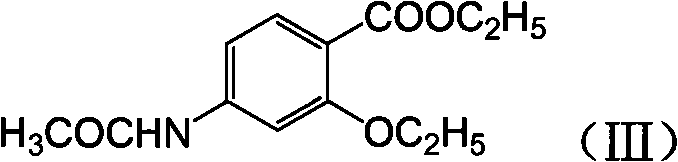
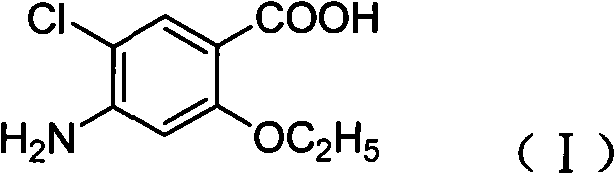Novel synthesis method and intermediate for 2-ethoxy-4-amino-5-chlorobenzoic acid
A technology of p-aminosalicylic acid and ethylation, applied in directions such as organic chemistry, can solve the problems of long preparation steps, high cost of raw materials, and high pollution, achieve high total yield, improve total yield, and shorten reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Steps: Preparation of acetamidosalicylic acid: Sodium p-aminosalicylate (52.54g, 0.3mol), water (260mL) and acetic anhydride (30.6g, 0.3mol) were added to the reaction flask, stirred and heated, and reacted at 50°C After 2 hours, the pH was adjusted to acidic after the reaction, filtered, washed with water, and dried to obtain p-acetamidosalicylic acid (47.73 g, 81.5%) as a white solid. mp 232°C. 1 H-NMR (400MHz, d 6 -DMSO): δ 2.07 (s, 3H), 7.02 (dd, J 1 =8.4Hz,J 2 =8.4Hz, 1H), 7.34(d, J=1.6Hz, 1H), 7.69(d, J=4.8Hz, 1H), 10.20(s, 1H), 11.35(br, 1H), 13.67(br, 1H ).
[0028] Step 2 Preparation of ethyl 2-ethoxy-4-acetamidobenzoate: p-acetamidosalicylic acid (46.84g, 0.24mol), DMF (500mL), potassium carbonate (66.34g, 0.48mol) and Ethyl bromide (65.38g, 0.6mol) was put into a reaction bottle, stirred and heated, reacted at 100°C for 8 hours, poured into water and stirred for crystallization, filtered, washed with water, dried to obtain a white solid 2-ethoxy-4-acetam...
Embodiment 2
[0032] Preparation of p-acetamidosalicylic acid: p-aminosalicylic acid (4.60g, 0.03mol), water (46mL) and acetic anhydride (3.07g, 0.03mol) were added to the reaction flask, stirred and heated, and reacted at 60°C for 4 hours, Cool, filter, wash with water, and dry to obtain white solid p-acetamidosalicylic acid (4.70 g, 80.2%).
Embodiment 3
[0034] Preparation of p-acetamidosalicylic acid: Potassium p-aminosalicylate (5.74g, 0.03mol), water (29mL) and acetic anhydride (3.07g, 0.03mol) were added to the reaction flask, stirred and heated, and reacted at 50°C for 2 hours , cooled, adjusted the pH to acidic, filtered, washed with water, and dried to obtain white solid p-acetamidosalicylic acid (4.76g, 81.3%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com