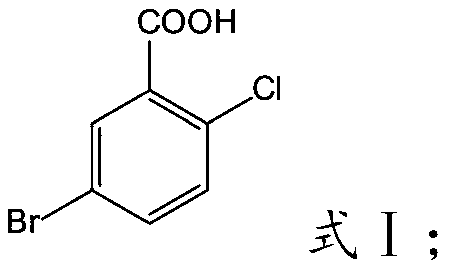
Preparation method of 5-bromo-2-chlorobenzoic acid
A technology of chlorobenzoic acid and bromine, applied in the field of preparation of 5-bromo-2-chlorobenzoic acid, can solve problems such as low yield, and achieve the effects of high yield, short steps and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention provides a kind of preparation method of 5-bromo-2-chlorobenzoic acid, comprises the following steps:
[0031] Mix 2-chlorobenzoic acid, bromine, catalyst and organic solvent for bromination reaction to obtain 5-bromo-2-chlorobenzoic acid.
[0032] In the present invention, unless otherwise specified, all raw materials are commercially available products well known to those skilled in the art.
[0033] In the present invention, the catalyst is preferably a Lewis acid, more preferably aluminum ferric chloride, ferric chloride, ferric tribromide, boron trifluoride, zinc chloride, niobium pentachloride and trifluoromethanesulfonate One or more of acid salts; when the catalyst is two or more of the above-mentioned specific selections, the present invention does not have any special restrictions on the proportioning of the specific substances.
[0034] In the present invention, the molar ratio of the 2-chlorobenzoic acid, bromine and catalyst is preferably 1:(...
Embodiment 1
[0049]Mix 93.6kg 2-chlorobenzoic acid and 750kg dichloromethane, add aluminum trichloride (162kg, each batch of 32.4kg) in 5 times under the condition of stirring, continue to stir for 30 minutes until aluminum trichloride is fully Dissolve, add 105.6kg bromine dropwise at 25°C for 30 minutes, react at room temperature for 48 hours, monitor the reaction by HPLC, terminate the reaction when the 2-chlorobenzoic acid content in the reaction solution is less than 0.5%;
[0050] Mix 200kg of dilute hydrochloric acid with a mass concentration of 30% and 400kg of deionized water, stir and cool down to -10°C, press the product system obtained after the bromination reaction into it for quenching, control the temperature below 0°C, and quench After completion, dichloromethane was removed by distillation under reduced pressure (stop when the internal temperature was 40°C), 600kg of toluene was added, the temperature was raised to 70-75°C to completely dissolve, the temperature was maintai...
Embodiment 2
[0053] Mix 46.8kg 2-chlorobenzoic acid and 375kg chloroform, add aluminum trichloride (81kg, each batch of 16.2kg) in 5 times under the condition of stirring, continue to stir for 30 minutes until aluminum trichloride is fully Dissolve, add 52.8kg bromine dropwise at 25°C for 30 minutes, react at room temperature for 48 hours, detect the reaction by HPLC, stop the reaction when the 2-chlorobenzoic acid content in the reaction solution is less than 0.5%;
[0054] Mix 100kg of dilute hydrochloric acid with a mass concentration of 30% and 200kg of deionized water, stir and cool down to -10°C, press the product system obtained after the bromination reaction into it for quenching, control the temperature below 0°C, and quench After completion, dichloromethane was removed by distillation under reduced pressure (stop when the internal temperature was 50°C), 300kg of toluene was added, the temperature was raised to 70-75°C until it was completely dissolved, and the temperature was main...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com