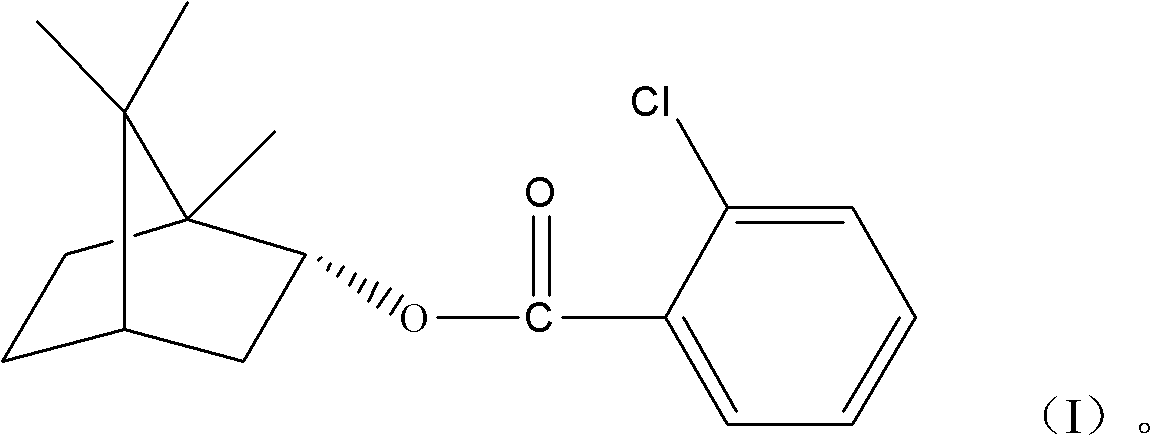
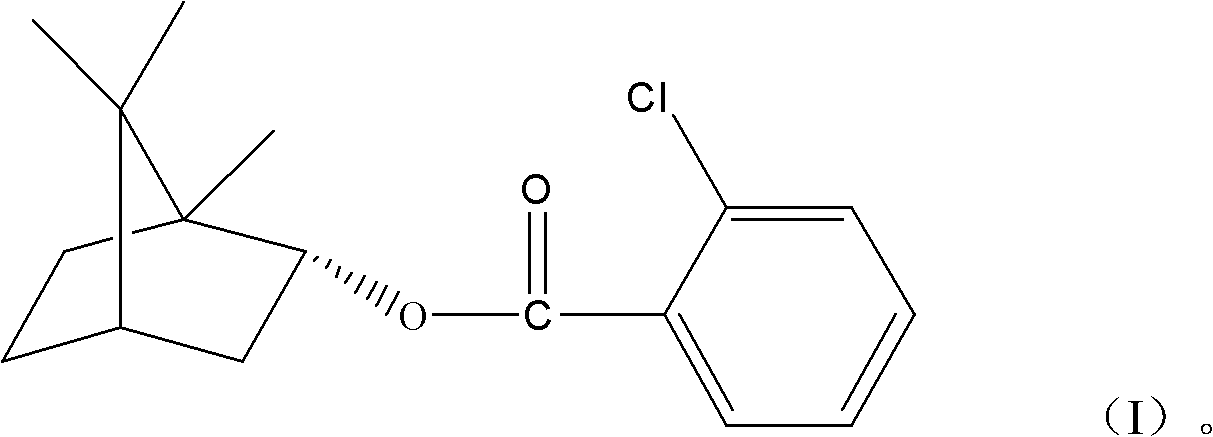
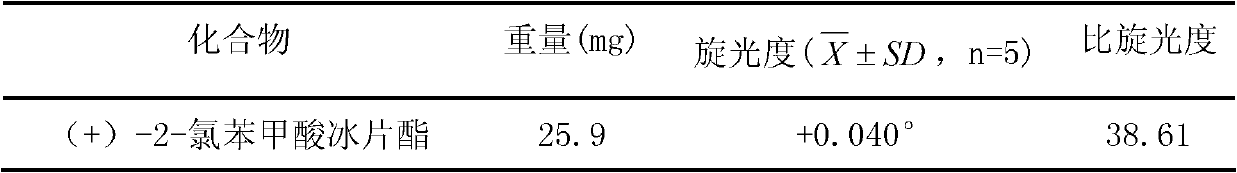
(+)-2-bornyl chlorobenzoate and its preparation method and application
A technology of chlorobenzoic acid and borneol, which is applied in the direction of nervous system diseases and drug combinations, etc., and can solve the problem that the ability of borneol to penetrate the blood-brain barrier is not ideal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] 1. Synthesis of (+)-2-chlorobornyl benzoate
[0014] Add 150g natural borneol, 156g 2-chlorobenzoic acid and 500ml toluene to a 1000ml round bottom flask; add 10g p-toluenesulfonic acid dropwise and mix well; react at 110°C for 6 hours. After the reaction, the catalyst is removed, and unreacted 2-chlorobenzoic acid and solvent are used to obtain the initial product. The initial product was recrystallized from ethanol to obtain white crystals.
[0015] 2. Properties of Bornylyl (+)-2-chlorobenzoate
[0016] The white crystals obtained in step 1 are hardly soluble in water, but soluble in acetone, ethyl acetate, ethanol, and methanol. The melting point is 41.4-42.2°C.
[0017] 3. Identification of the compounds of the present invention:
[0018] 1. Elemental analysis: Elemental analysis (%): measured value C69.70, H7.20 calculated value C69.73, H7.23.
[0019] 2. NMR spectrogram analysis: German Bruker 500MHz nuclear magnetic resonance instrument, model AV-500; probe...
Embodiment 2
[0035] 1. Acute toxicity test of dextroborneol in mice
[0036] 1.1. Experimental animals
[0037] 60 Kunming white mice, half male and half male, body weight 20±2g. Provided by the Guangdong Provincial Laboratory Animal Center, certificate number: SCXK Yue 2008-0001 Yue Jian Zheng Zi 2007A013, used for the test after 3 days of adaptation.
[0038] 1.2. Experimental method
[0039] Mice were fasted overnight for 15 h before administration, and had free access to water. They were divided into 6 groups with 10 rats in each group. Groups 1 to 5 were given dextroborneol blended oil solution, and the dosages were 6170, 4319, 3023, 2116, 1481 mg / kg; Blend oil. According to the weight of the mice, the drug group was fed with dextroborneol at a dose of 0.2 mL / 10 g, and the control group was fed with the same volume of edible oil. Return to normal feeding 2 hours after administration, observe continuously for 14 days, observe the changes in the weight of the mice every day, whethe...
Embodiment 3
[0057] (+)-2-Chlorobenzoic acid bornyl ester permeabilization experiment of blood-brain barrier
[0058] Test animals: Kunming mice, weighing 18-23g, half male and half male.
[0059] Experimental method: Kunming mice were randomly divided into two groups, with 4 mice in the blank group and 12 mice in the test group. They were fasted the night before the experiment. According to the body weight of the mice, a suspension of bornyl 2-chlorobenzoate and 0.5% (v / v) Tween was prepared at a dose of 1.5 g / kg. Mice in the blank group and the test group were fed with 0.4ml / 20g (body weight) of 0.5% (v / v) Tween solution, 2-chlorobenzoate bornyl ester and 0.5% (v / v) Tween suspension respectively. . Each group of mice was injected with 0.4% 0.5% (m / m) Evans blue solution by 10ml / kg (body weight) tail vein 25min after the drug administration, and after 2min, the brain was decapitated and the brain was washed with a small amount of normal saline. The filter paper was blotted dry, and aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com