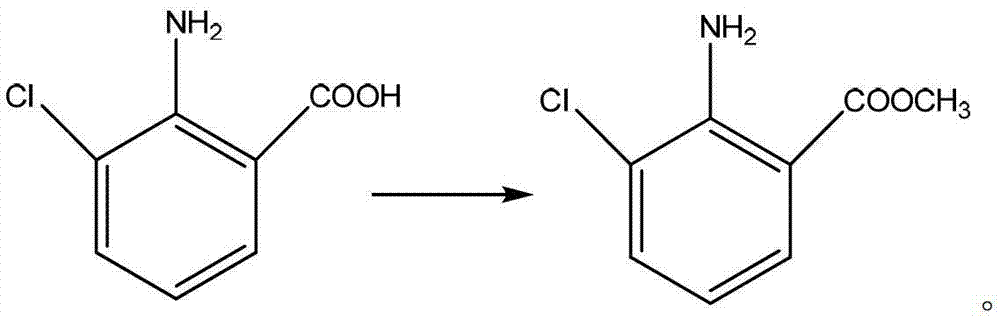
Preparation method of 2-amino-3-chlorobenzoic methyl ester
A technology of methyl chlorobenzoate and chlorobenzoic acid is applied in the field of preparation of herbicide intermediates, and can solve the problems of complex reaction process, difficult control, and difficulty in obtaining, and achieves simple reaction process, easy operation, and improved yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0020] ①At room temperature, add 150g of DMF to a 500mL reaction flask, then add 30g of 2-amino-3-chlorobenzoic acid (0.175mol), and then add 17.3g of potassium carbonate (0.125mol).
[0021] ②After the addition, cool to 10°C and stir for 0.5h, then slowly add 22.4g of dimethyl sulfate (0.178mol) dropwise.
[0022] ③After dripping, rise to room temperature and stir the reaction for 6h.
[0023] ④The reacted material was poured into 400mL water, and a white solid was separated out. After stirring for 1h, filter, and the filter cake was washed with water and dried to obtain 31.8g of 2-amino-3-chlorobenzoic acid methyl ester, the yield was 95.0%, the purity 97% (HPLC).
Embodiment 2~ Embodiment 4)
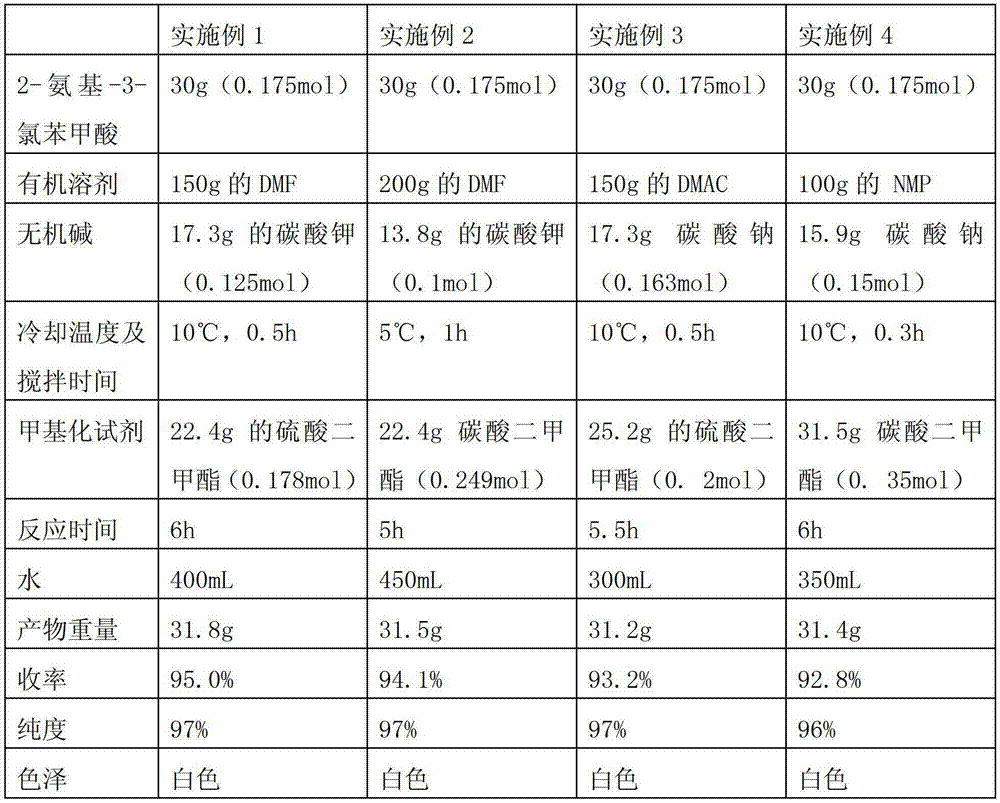
[0025] The preparation method of each embodiment is basically the same as that of Example 1, and the differences are shown in Table 1.
[0026] Table 1
[0027]
[0028] .
[0029] As can be seen from Table 1, the effect of adopting potassium carbonate as the inorganic base is slightly better than that of sodium carbonate, and the effect of adopting dimethyl sulfate as the methylating agent is also slightly better than that of dimethyl carbonate.
Embodiment 5)
[0031] ①At room temperature, add 150g of DMF to a 500mL reaction flask, then add 30g of 2-amino-3-chlorobenzoic acid (0.175mol), and then add 17.3g of potassium carbonate (0.125mol).
[0032] ②After the addition, cool to 10°C and stir for 0.5h, then slowly add 22.4g of dimethyl sulfate (0.178mol) dropwise.
[0033] ③After dripping, rise to room temperature and stir the reaction for 6h.
[0034] ④The reacted material was directly filtered, and the filter cake was washed with water and dried to obtain 30.8 g of methyl 2-amino-3-chlorobenzoate, with a yield of 91.1% and a purity of 96% (HPLC).
[0035] From Example 1 and Example 5, it can be seen that in step ④, pouring the reacted material into water is conducive to the complete precipitation of the product, so that the yield is higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com