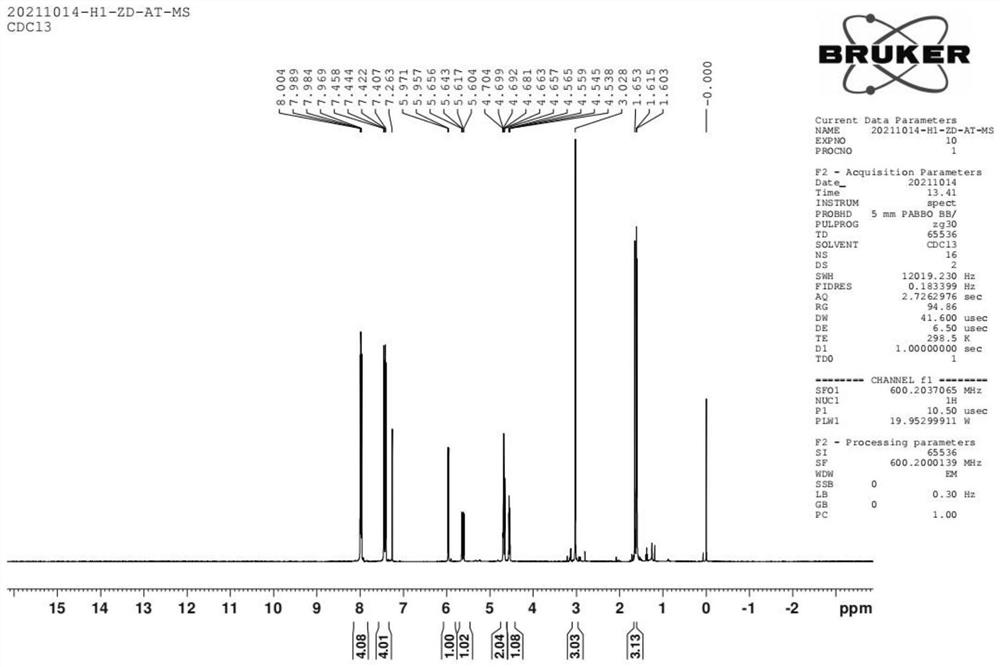
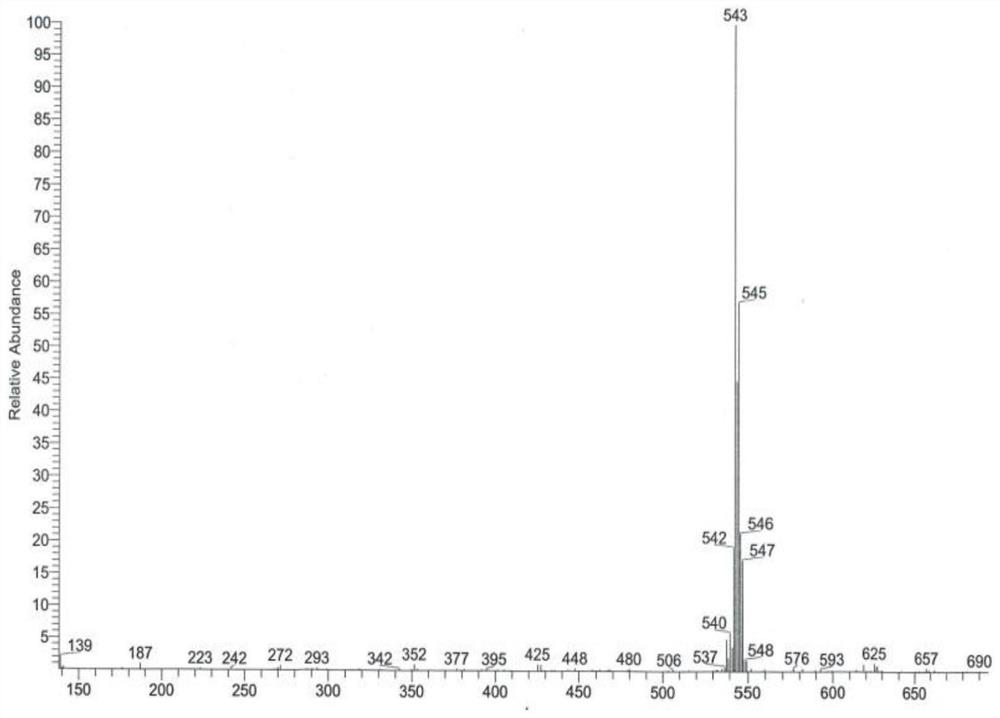
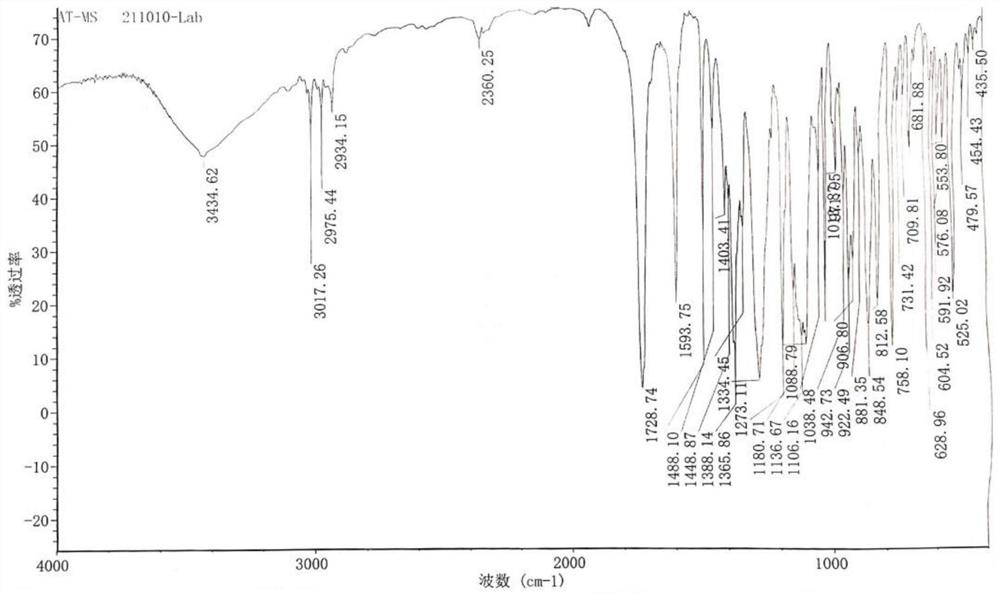
Preparation method of alpha-configuration aroyl bromo-sugar
A technology of aroyl bromosugar and configuration, which is applied in the field of preparation of aroyl bromosugar, and can solve problems such as unguaranteed yield of aroyl bromosugar and unfavorable α-configuration aroyl bromosugar yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The invention provides a method for preparing an aroyl bromosugar of α configuration, comprising the following steps:
[0033] Under the condition of acid-binding agent, (2R)-3,5-bis(4-chlorobenzoate)-2-deoxy-2-fluoro-2-methyl -β-D-erythro-pentofuranose is mesylated to obtain a mixture containing α-sulfonylated sugars and β-sulfonylated sugars;
[0034] Transforming the mixture containing α-sulfonylated sugars and β-sulfonylated sugars to obtain a mixture mainly containing β-sulfonylated sugars;
[0035] The mixture mainly composed of β-sulfonylated sugars is subjected to S N The configuration of 2 is inverted to give the aroyl bromosugar in the alpha configuration.
[0036] In the present invention, unless otherwise specified, the raw materials used in the present invention are preferably commercially available products.
[0037] In the present invention, under the condition of an acid-binding agent, (2R)-3,5-bis(4-chlorobenzoate)-2-deoxy-2-fluoro-2- Methyl-β-D-ery...
Embodiment 1
[0070] The preparation of the pure product of embodiment 1β-sulfonylated sugar
[0071] (2R)-3,5-bis(4-chlorobenzoate)-2-deoxy-2-fluoro-2-methyl-β-D-erythro-pentofuranose (44.3g , 100mmol) was dissolved in dichloromethane (222g), and triethylamine (11.1g, 110mmol) was added to cool down to -50°C, and the methanesulfonyl chloride solution was added dropwise in 1.5h (the methanesulfonyl chloride solution included methanesulfonyl chloride (12.0 g, 105mmol), dichloromethane (88.6g)), the dropwise temperature is not higher than -50°C. Incubate after dropping, and monitor the β-configuration (2R)-3,5-bis(4-chlorobenzoate)-2-deoxy-2-fluoro-2-methyl-β-D-erythro- The mass content of pentofuranose is less than 1%, and the mesylation reaction is completed; at this time, HPLC monitors that the mass proportion of β-sulfonylated sugar in the reaction solution is 85%, and the mass proportion of α-sulfonylated sugar is 15%. Add 200g of water and 10g of sodium bicarbonate to the mesylation ...
Embodiment 2
[0079] Preparation of pure aroyl bromosugar of embodiment 2α configuration
[0080]Dissolve the pure β-sulfonylated sugar obtained in Example 1 (52.1 g, 100 mmol) in acetonitrile, cool down to 8°C, add anhydrous lithium bromide (13.1 g, 150 mmol) to react for 12 hours, and detect the β-sulfonylated sugar by HPLC The content of sugar is less than 1%, the solvent is evaporated to dryness, and dichloromethane 300g and water 200g are added, and the first extraction is carried out to obtain the first aqueous phase and the first organic phase; the first organic phase is mixed with 200g water, and the The second extraction obtains the second aqueous phase and the second organic phase; the second organic phase is mixed with 200g water, and the third extraction is carried out to obtain the third aqueous phase and the third organic phase; the third organic phase As the organic phase obtained by extraction, dichloromethane in the organic phase was evaporated to dryness; the sticky substa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com