Preparation method of O-diphenylphosphine benzoic acid
A technology of diphenylphosphine and benzoic acid is applied in the field of preparation of organic phosphine ligands, which can solve the problems of consuming sodium diphenylphosphine, low yield, low yield and the like, so as to improve reaction yield and reduce production Cost, effect of suppressing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0019] The preparation method of the O-diphenylphosphine benzoic acid of the present embodiment has the following steps:
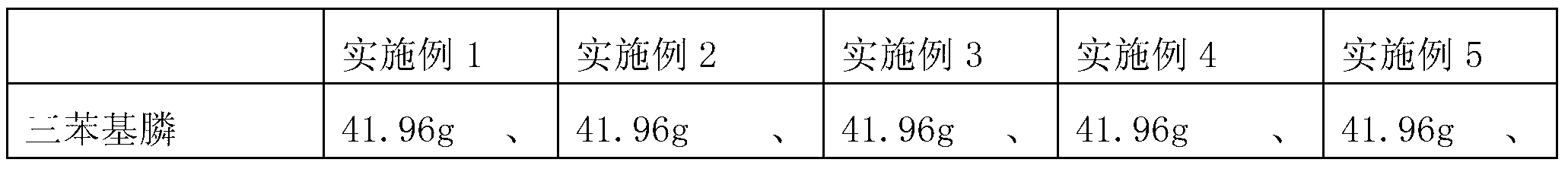
[0020] ①Under the protection of nitrogen, add 2.24g of lithium metal (0.32mol), 41.96g of triphenylphosphine (0.16mol) and 320mL of 2-methyltetrahydrofuran organic solvent into a 1L three-necked flask. After 5min, the reaction system The color turns red with slight exotherm, and the cracking reaction is carried out at 25±2°C for 3 hours, the color of the solution turns dark red, the metal lithium disappears completely, and diphenylphosphinelithium and phenyllithium are generated.
[0021] ②At a temperature of 25±2°C, slowly drop 16.2g of diisopropylamine (0.16mol) into a three-necked flask within 30min~60min, and keep it warm until the phenyl lithium reaction in the reaction system monitored by HPLC is complete .
[0022] ③Under the protection of nitrogen, cool in an ice-water bath to 5±2°C, and slowly drop 27.3g of methyl O-chlorobenzoate (0.16mol) into ...
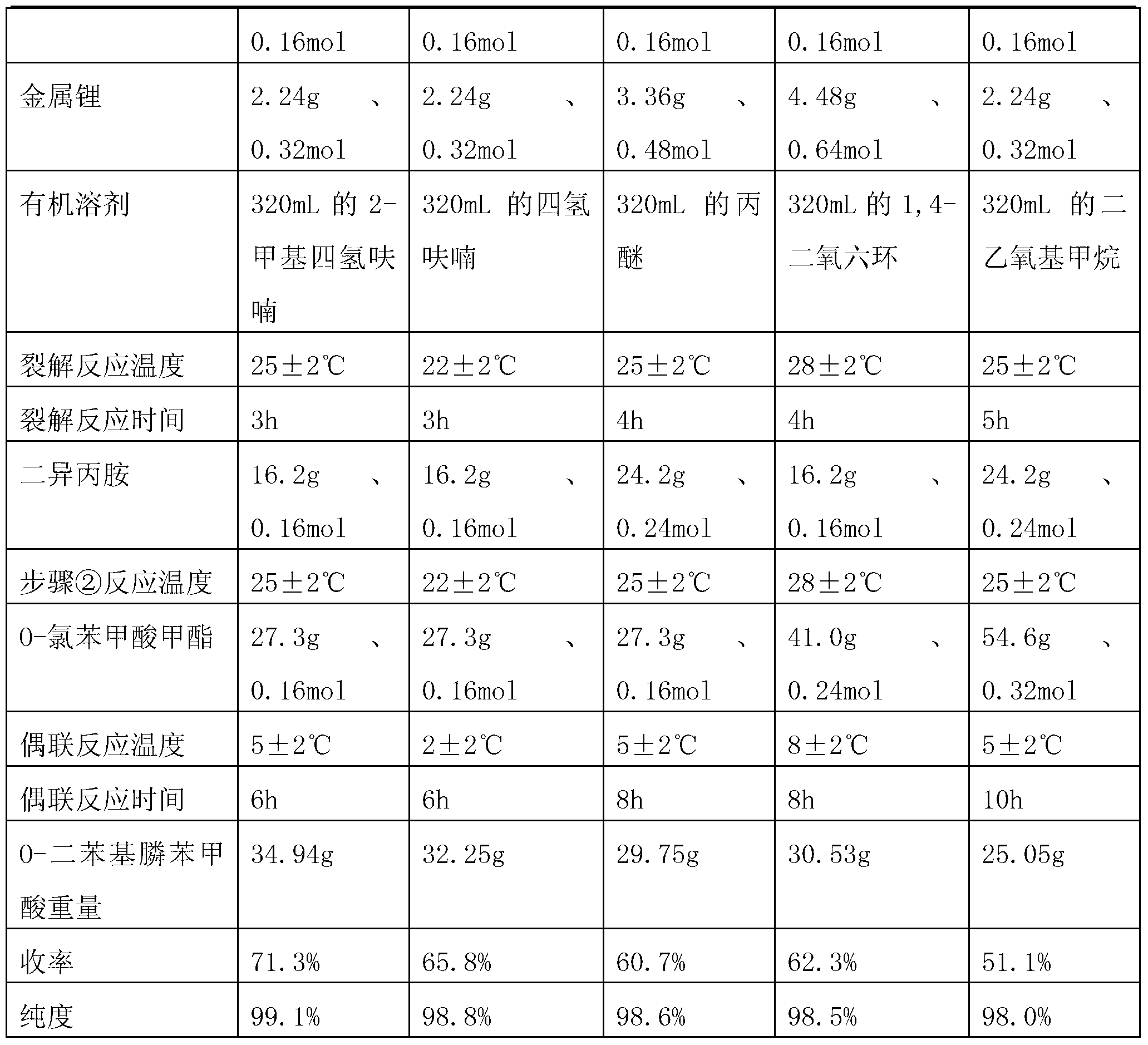
Embodiment 2~ Embodiment 5)
[0026] The preparation method of each embodiment is basically the same as that of Example 1, except that see Table 1.
[0027] Table 1
[0028]
[0029]
[0030] .
Embodiment 6)
[0032] The preparation method of the present embodiment is basically the same as that of Example 1, except that step ④:
[0033] ④ After the reaction, the reaction liquid was evaporated to remove the solvent under reduced pressure, and then 48 g of 20 wt % sodium hydroxide aqueous solution (containing 0.24 mol of sodium hydroxide) was added for hydrolysis under reflux for 3 h.
[0034] The hydrolyzed reaction solution was washed with 50 mL of dichloromethane, separated, and the organic layer was discarded (repeated washing twice).
[0035] The aqueous layer was acidified and neutralized with hydrochloric acid with a concentration of 36 wt%, and a pale yellow solid was precipitated. After purification with methanol, 34.84 g of O-diphenylphosphine benzoic acid was obtained, with a yield of 71.1% and a purity of 99.6% (HPLC).
[0036] As can be seen from Example 1 and Example 6, the purity of the hydrolyzed reaction solution after washing with dichloromethane can be increased by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com