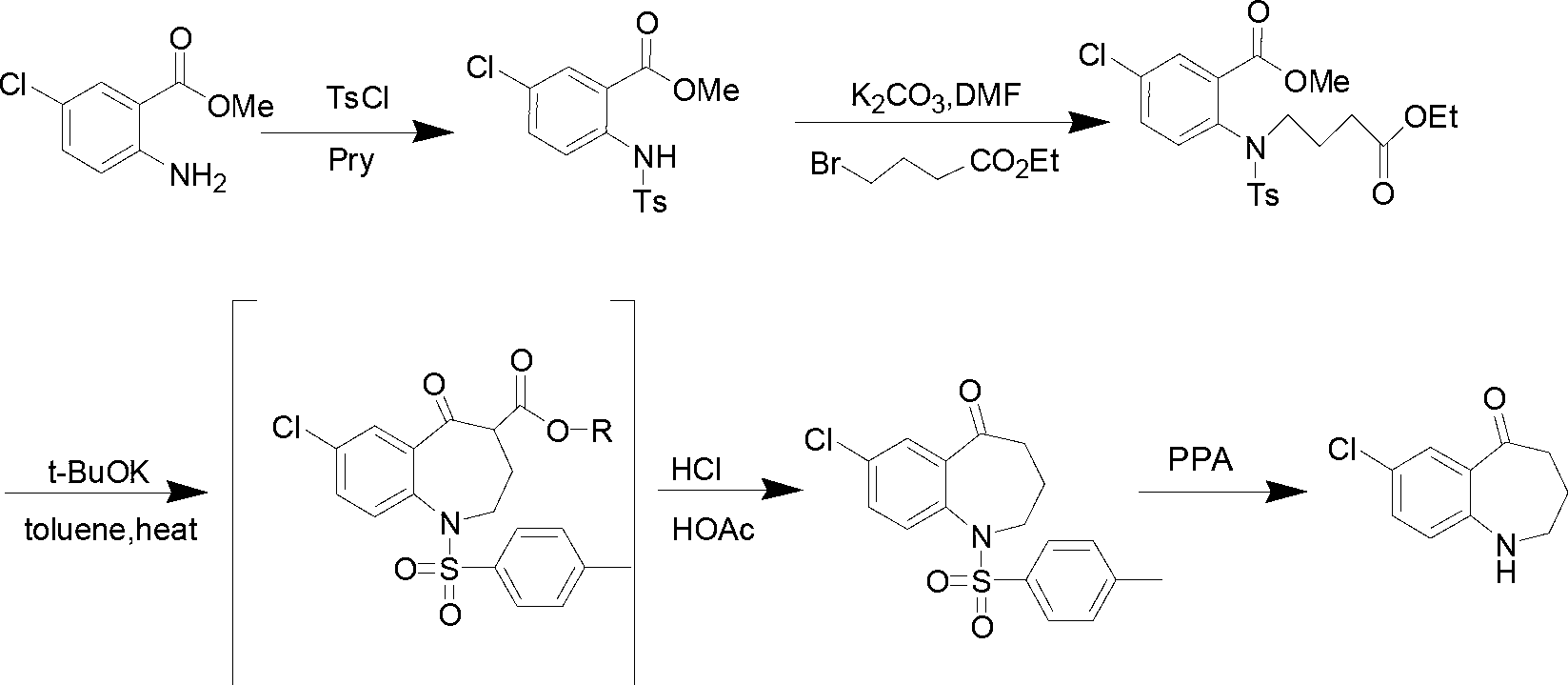
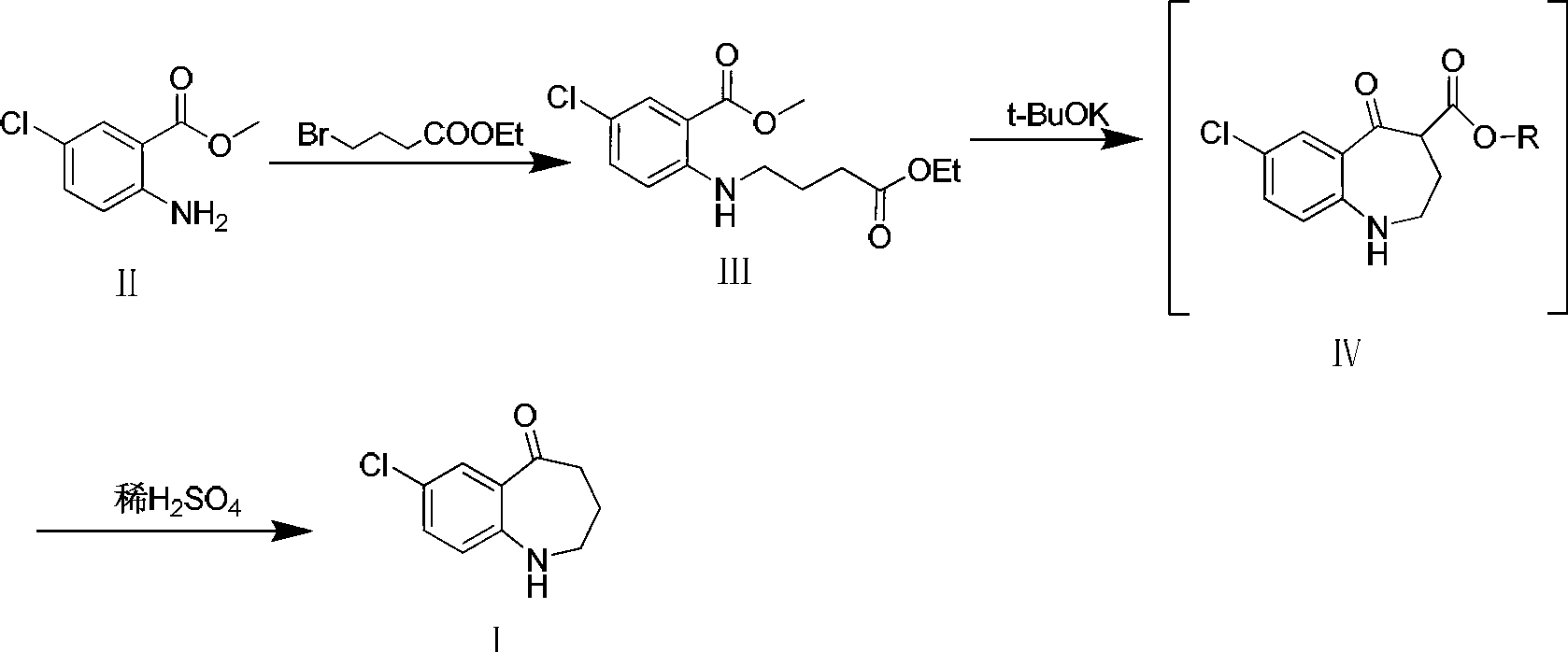
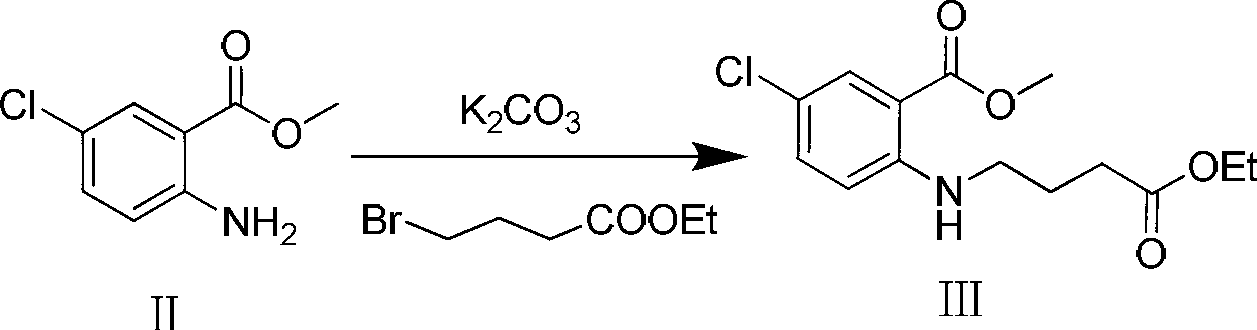
Preparation method of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine
A technology of benzonitride and oxo, which is applied in the direction of organic chemistry, etc., can solve the problems of cumbersome experimental operation, unsatisfactory yield, and increased reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
[0017] Add compound II (10g, 53.9mmol) and potassium carbonate (14.9g, 107.8mmol) into a 250ml reaction flask, add 100ml of DMF and stir to dissolve. At -5°C, ethyl 4-bromobutyrate (10.5g, 53.9 mmol) in DMF (50ml) was slowly added dropwise to the reaction solution. After the dropwise addition, react at -5°C for 1 h, then raise the temperature to 60°C for 1 h. TLC [developing solvent: ethyl acetate-petroleum ether (1:5), the same below] After the detection reaction is complete, the reaction solution is poured into ice water, a yellow solid is precipitated, and the solid is filtered out. The obtained yellow solid was dissolved in dichloromethane, washed with saturated brine (15ml×3), the organic layers were combined, dried over anhydrous sodium sulfate, and left overnight. After filtration, the solvent was evaporated under reduced pressure to obtain a pale yellow solid (13.8 g, 85.2%) with a purity of 93.0% (HPLC normalization method).
Embodiment 2
[0019]
[0020] Compound II (10g, 53.9mmol) and triethylamine (10.9g, 107.8mmol) were added to a 250ml reaction flask, 100ml of dichloromethane was added and stirred to dissolve, at -10°C, ethyl 4-bromobutyrate ( 10.5g, 53.9mmol) of dichloromethane solution (50ml) was slowly added dropwise to the reaction solution. After the dropwise addition, react at -10°C for 1 h, then raise the temperature to reflux for 3 h. After the reaction was complete as detected by TLC, the reaction solution was poured into 100 ml of ice water, shaken to separate layers, and several layers were separated, and washed with water three times in a row. The organic layer was dried over anhydrous sodium sulfate and left overnight. It was filtered and evaporated to dryness under reduced pressure to obtain a light yellow solid (13.4 g, 83.0%) with a purity of 92.4% (HPLC normalization method).
Embodiment 3
[0022]
[0023] Add compound II (10g, 53.9mmol) and sodium hydroxide (3.2g, 80.8mmol) to a 250ml reaction flask, add 100ml tetrahydrofuran and stir to dissolve, and at -15°C, ethyl 4-bromobutyrate (10.5g , 53.9 mmol) in tetrahydrofuran (50 ml) was slowly added dropwise to the reaction solution. After the dropwise addition, react at -15°C for 1 h, then raise the temperature to 30°C for 2 h. After the completion of the reaction as detected by TLC, the reaction solution was poured into ice water, a yellow solid was precipitated, and the solid was filtered out. The obtained yellow solid was dissolved in dichloromethane, washed with saturated brine (15ml×3), the organic layers were combined, dried over anhydrous sodium sulfate, and left overnight. After filtration, the solvent was evaporated under reduced pressure to obtain a light yellow solid (14.4 g, 89.1%) with a purity of 95.1% (HPLC normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com