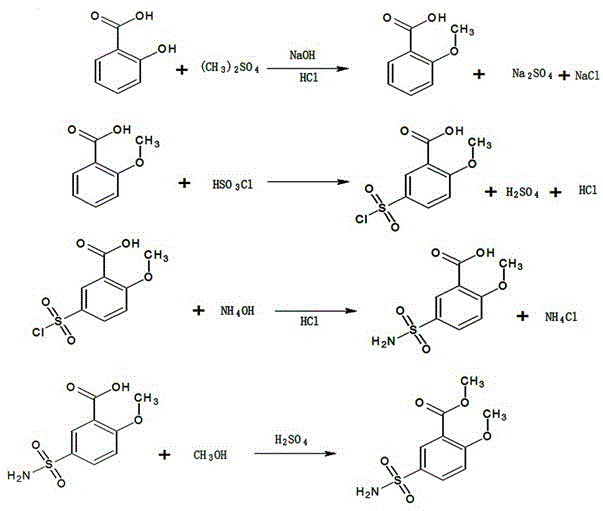
Method for synthesizing 2-methoxy-5-aminosulfonylmethyl benzoate by one-step method
A technology of methyl sulfamoylbenzoate and methyl chlorobenzoate, which is applied in the field of organic compound synthesis, can solve the problems of high processing cost, large amount of three wastes, long process route, etc., and achieve high yield, good quality and process short process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
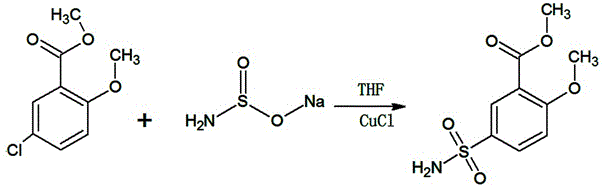
[0016] Such as figure 2 In the synthetic route shown, 300g of tetrahydrofuran, 50g (0.25mol) of methyl 2-methoxy-5-chlorobenzoate, and 1.25g of cuprous chloride (0.0125mol) will be added to a 1000ml reaction bottle equipped with a reflux device , 25.7g (0.25mol) sodium sulfinate, heated to 45 ° C, and kept at this temperature for 12 hours, after the heat preservation was completed, 2 grams of activated carbon was added to the reaction solution and filtered under heat, and the filtrate was concentrated to dryness under reduced pressure, 60 It was dried under vacuum at ℃ to obtain 57.9 g (0.236 mol) of white crystalline powder of methyl 2-methoxy-5-sulfamoylbenzoate, with a yield of 94.5% and a content of 99.51% (HPLC).
[0017] Among them, HPLC detection conditions: mobile phase: 700 milliliters of water; 200 milliliters of methanol. Detection wavelength: 240nm, flow rate 1.0ml / min, sample 0.01g, dilute to 25ml with mobile phase, injection volume 5μl.
Embodiment 2
[0019] Such as figure 2 In the synthetic route shown, 300g of tetrahydrofuran, 50g (0.25mol) of methyl 2-methoxy-5-chlorobenzoate, and 2.5g (0.025mol) of cuprous chloride will be added to a 1000ml reaction bottle equipped with a reflux device , 26.8g (0.26mol) sodium sulfinate, heated to 50 ° C, and kept at this temperature for 16 hours, after the heat preservation was completed, 2 grams of activated carbon was added to the reaction solution and filtered under heat, and the filtrate was concentrated to dryness under reduced pressure, 60 It was dried under vacuum at ℃ to obtain 58.3 g (0.238 mol) of white crystalline powder of methyl 2-methoxy-5-sulfamoylbenzoate, yield 95.09%, content 99.66% (HPLC).
[0020] Among them, HPLC detection conditions: mobile phase: 700 milliliters of water; 200 milliliters of methanol. Detection wavelength: 240nm, flow rate 1.0ml / min, sample 0.01g, dilute to 25ml with mobile phase, injection volume 5μl.
Embodiment 3
[0022] Such as figure 2 In the synthetic route shown, 300g of tetrahydrofuran, 50g (0.25mol) of methyl 2-methoxy-5-chlorobenzoate, 2g (0.02mol) of cuprous chloride will be added to a 1000ml reaction flask equipped with a reflux device, 28.3g (0.275mol) sodium sulfinate, heat up to 60°C, and keep at this temperature for 8 hours. After the heat preservation is over, add 2 grams of activated carbon to the reaction solution and filter it under heat. The filtrate is concentrated under reduced pressure to dryness, After vacuum drying, 59.2 g (0.241 mol) of white crystalline powder of methyl 2-methoxy-5-sulfamoylbenzoate was obtained, with a yield of 96.55% and a content of 99.51% (HPLC).
[0023] Among them, HPLC detection conditions: mobile phase: 700 milliliters of water; 200 milliliters of methanol. Detection wavelength: 240nm, flow rate 1.0ml / min, sample 0.01g, dilute to 25ml with mobile phase, injection volume 5μl.
[0024] As can be seen from Examples 1-3, the advantages of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com