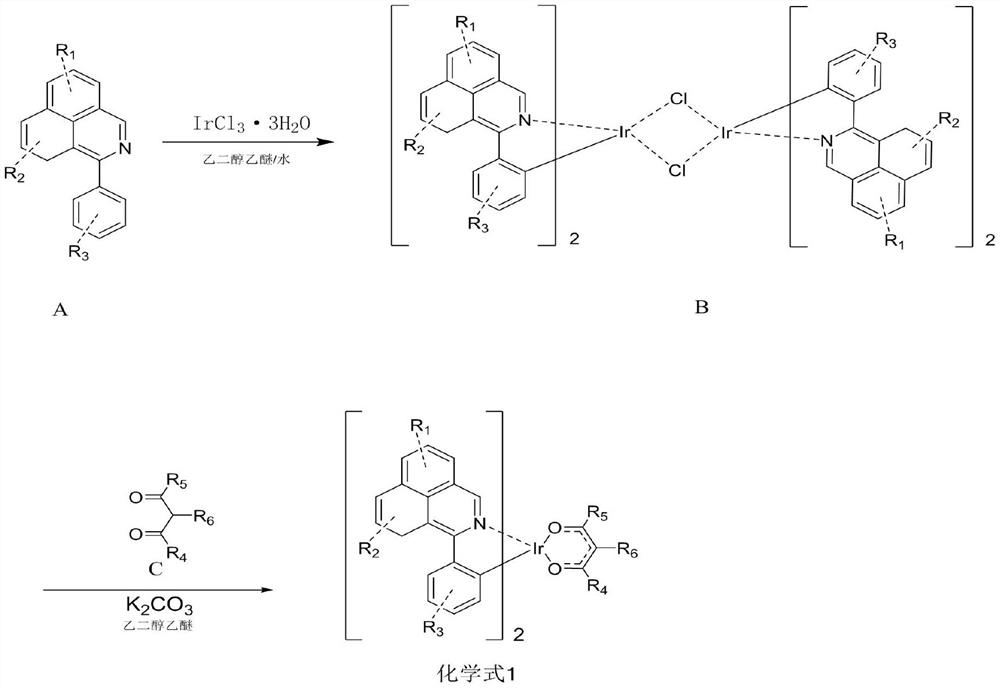
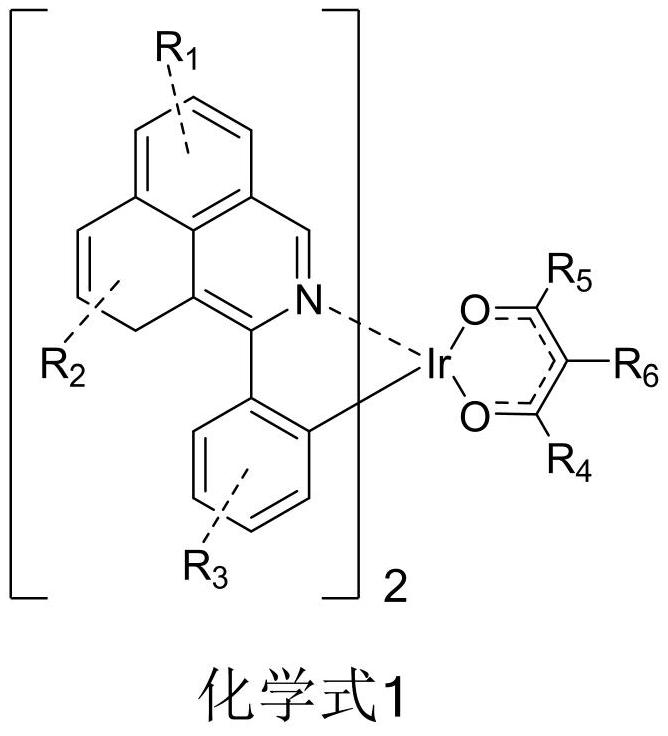
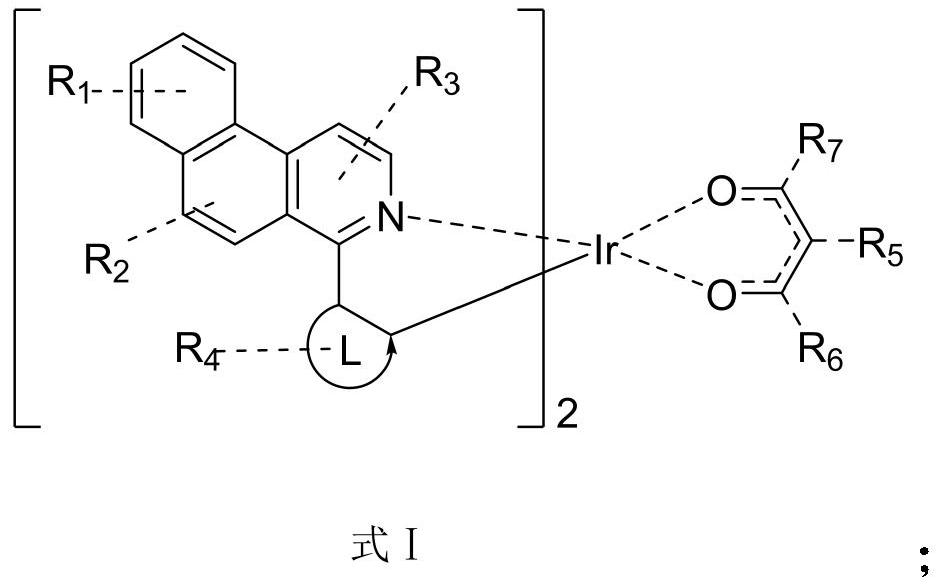
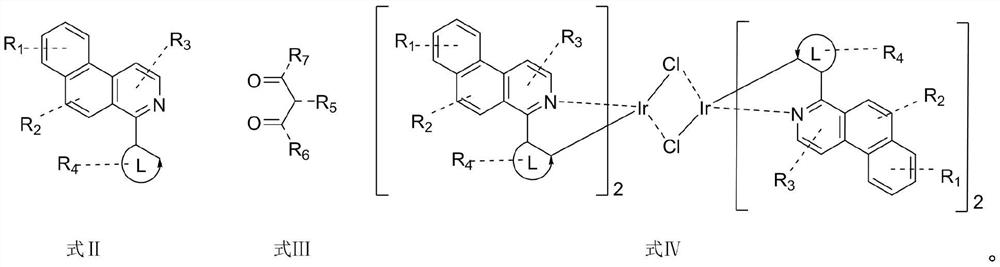
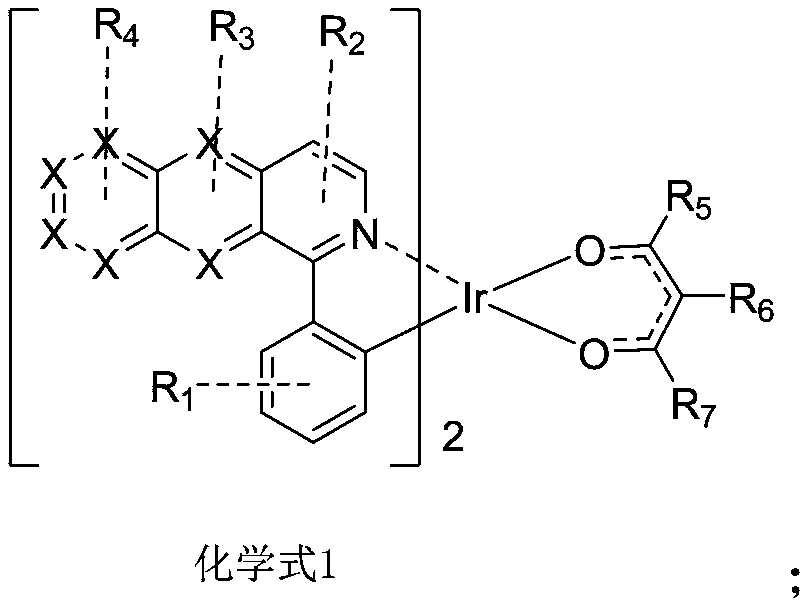
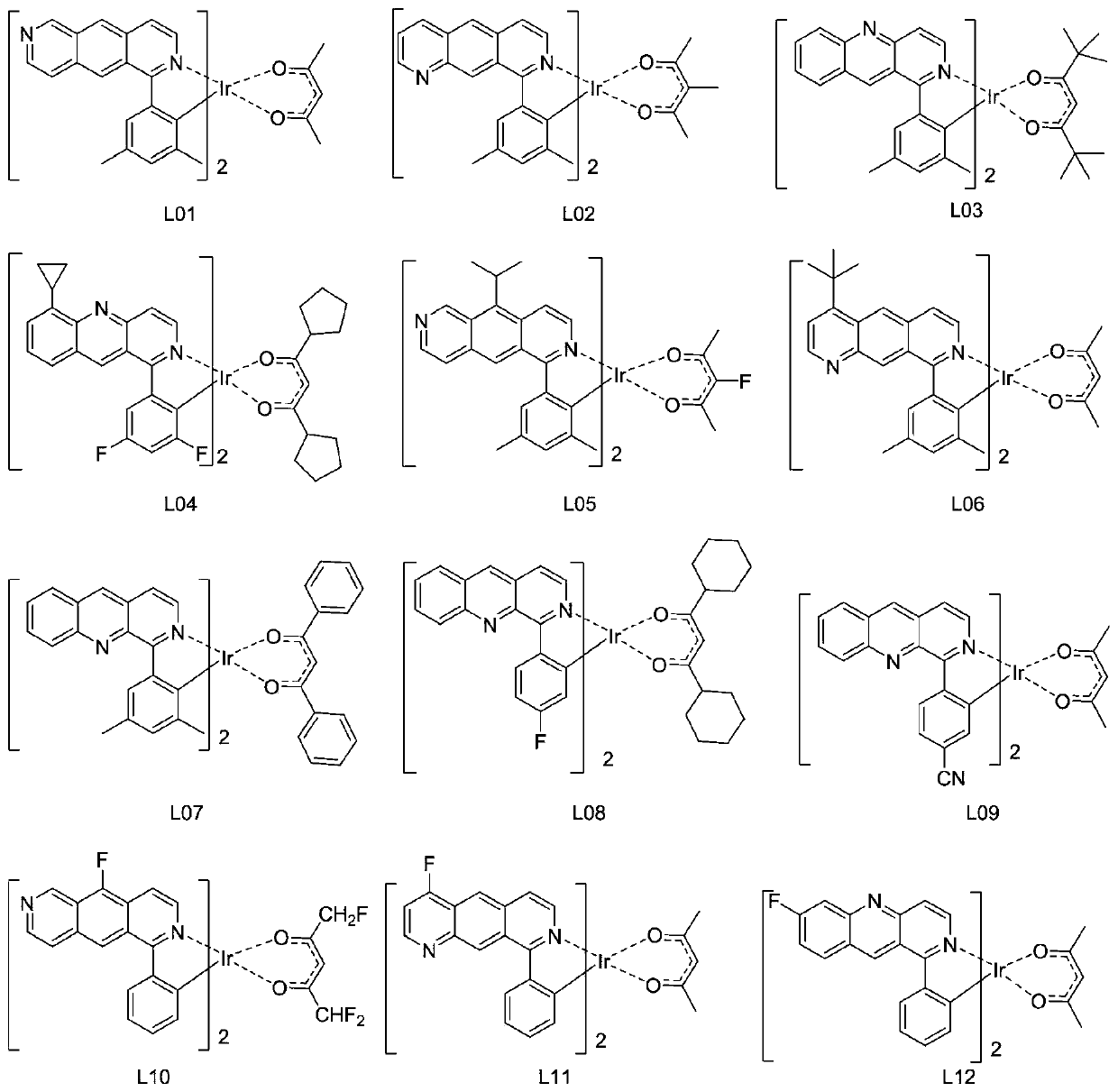
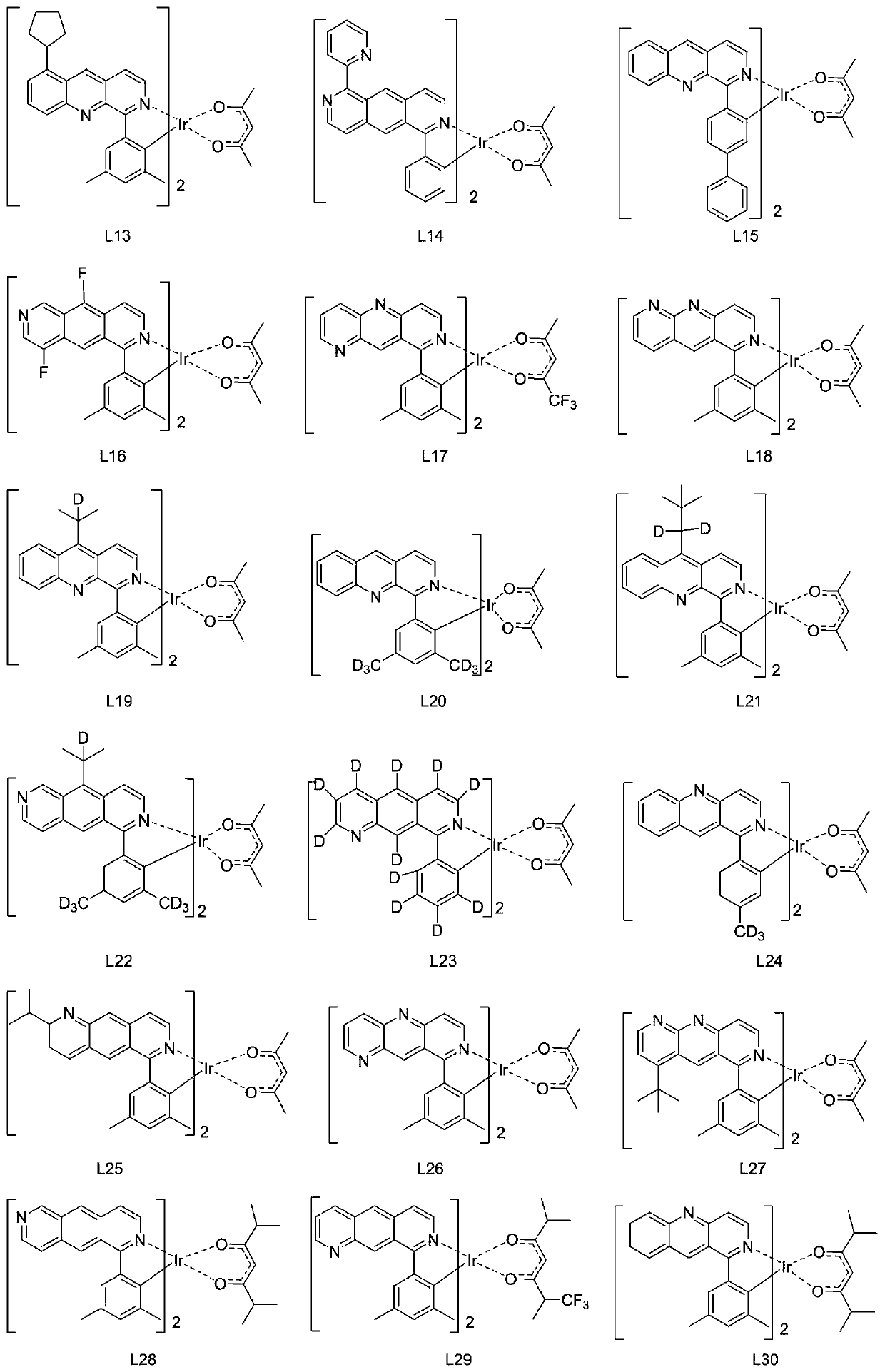
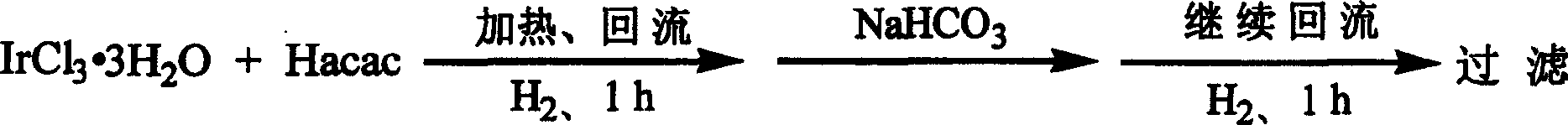Patents
Literature
50 results about "Iridium(III) chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Iridium(III) chloride is the inorganic compound with the formula IrCl₃. The anhydrous compound is relatively rare, but the related hydrate is useful for preparing other iridium compounds. The anhydrous salt is a dark green crystalline solid. More commonly encountered is the trihydrate IrCl₃(H₂O)₃.
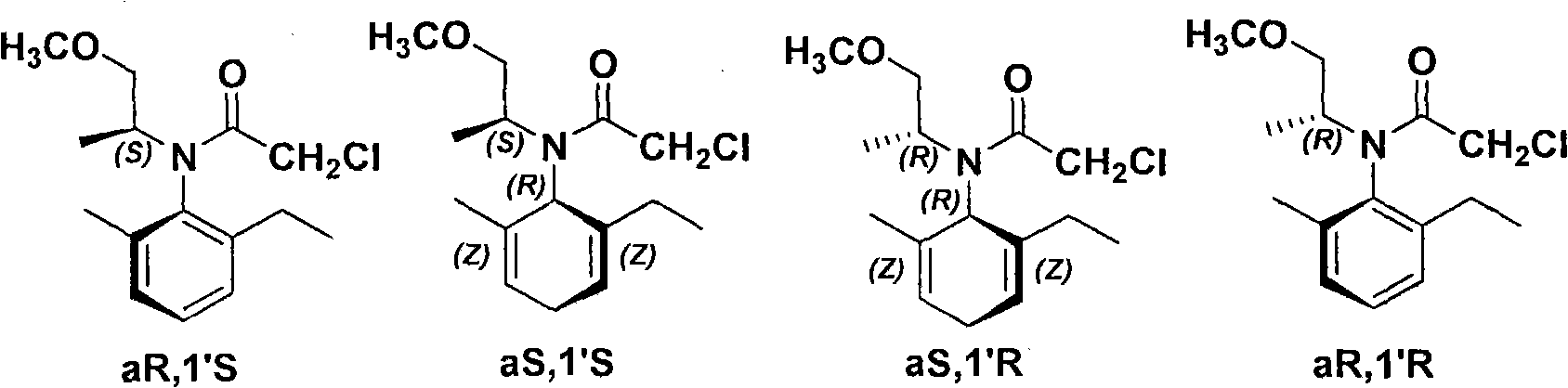
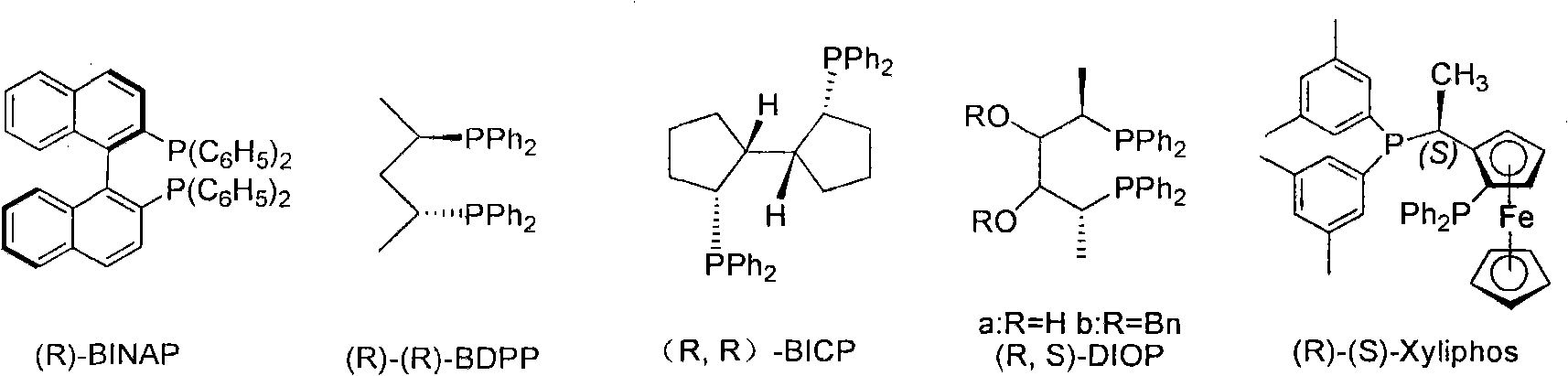
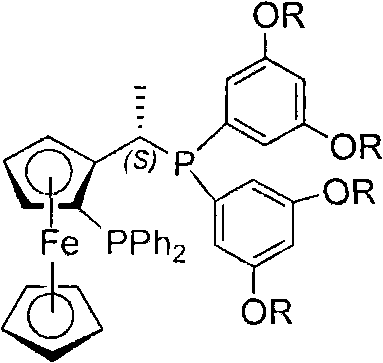
Chiral diphosphite ligand and iridium composite catalyst and preparation thereof method and application to asymmetrical hydrogenization synthesis (S)-metolachlor
ActiveCN101857612AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneDiphosphines
The invention relates to a kind of chiral diphosphite ligands, an iridium composite catalyst thereof, a preparation method and application thereof. The ligands are obtained through using chiral (R)-(S)-1-dimethylamino ethyiferroene as raw materials to react with diphenyl phosphonium chloride under the effect of butyl lithium and then to carry out displacement reaction with diaryl phosphine alkane. The chiral diphosphite ligands respectively act with homotropilidene compositions of iridous chloride, tetrabutyl ammonium iodide and glacial acetic acid, and imine asymmetrical hydrogenization catalysts can be obtained. When the iridium-diphosphine catalysts are used for catalyzing 2-methyl-6-ethyl-N-methylene aniline (EMA-imine) hydrogenization reaction, (S)-N-(1-anisyl-2-propyl)-2-methyl-6- ethylaniline ((S)-NNA) can be obtained, and the antimer excessive value (ee) can reach 86.5 percent. The (S)-NNA and chloracetyl chloride carry out acylation reaction to obtain (S)-metolachlor with the ee value of 86 percent. Thereby, the ligands provided by the invneiton can be used for synthesizing chiral herbicidal chemicals of (S)-metolachlor.
Owner:NANJING UNIV OF TECH +2
Method for low temperature aqueous solution electrochemical codeposition of nickel iridium alloy
The invention relates to a method for low temperature aqueous solution electrochemical codeposition of nickel iridium alloy, and the method is characterized in that: nickel salts comprise 0.001-0.1mol / L of nickel sulfate, 0.035-0.05mol / L of nickel chloride and 0.005-0.5mol / L of nickel sulfamate, iridium salt is 0.005-0.1mol / L of trichloride iridium containing water crystal, the complex is 0.1-0.5mol / L of citric acid, additives comprise 0.001-0.01mol / L of saccharin, 0.0005-0.01mol / L of gelatin, 0.0001-0.005mol / L of lauryl sodium sulfate, 0.0001-0.005mol / L of naphthyldisulfnate, 0.0001-0.005mol / L of coumarin and 0.0001-0.005mol / L of paratoluene sulfonamide, and the nickel salts, the iridium salt, the complex and the additives are dissolved in deionized water; the plating solution pH is 2-12, deposition temperature is 25-90 DEG C, the current density is 20-100mA / cm<2>, agitation and nitrogen protection are needed in the deposition process, stirring speed of a stirrer is 10-1000 rpm, electrochemical codeposition of nickel iridium alloy can be performed on the surface of an electrode of a pretreated workpiece, and nickel iridium alloy having the advantages of homogeneous composition, fine crystalline grains, good bonding and excellent corrosion resistance can be obtained, the iridium content is 0.1-50at%, and the alloy thickness is 0.1 to 100 mu m.
Owner:CHANGZHOU UNIV
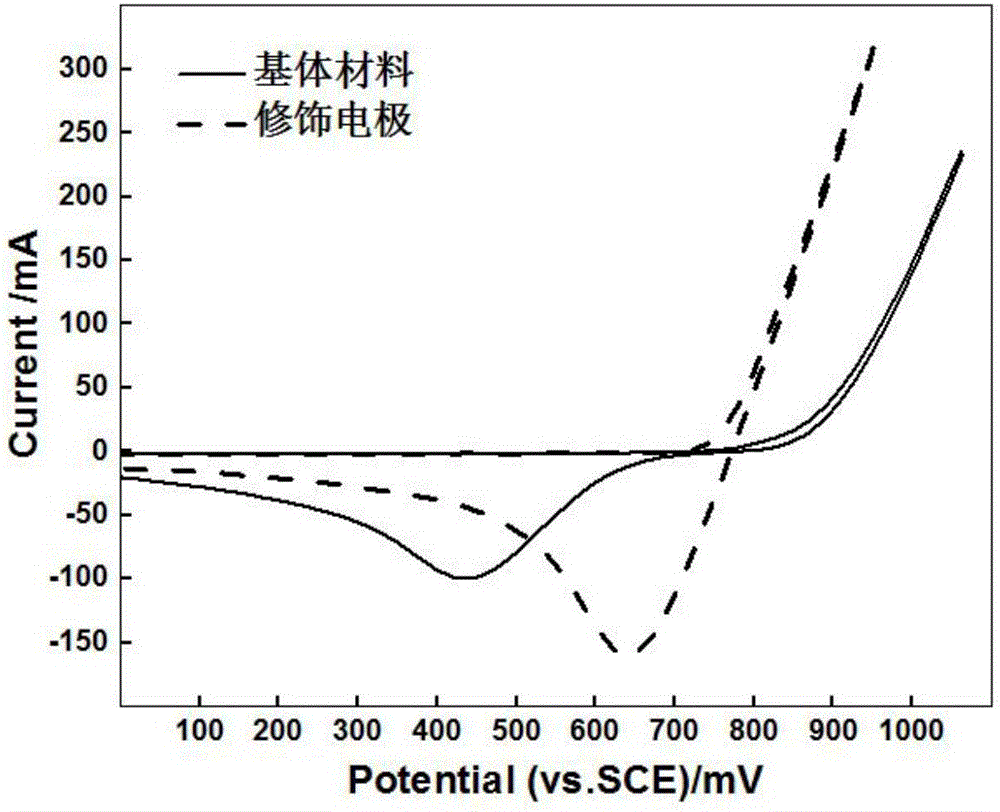
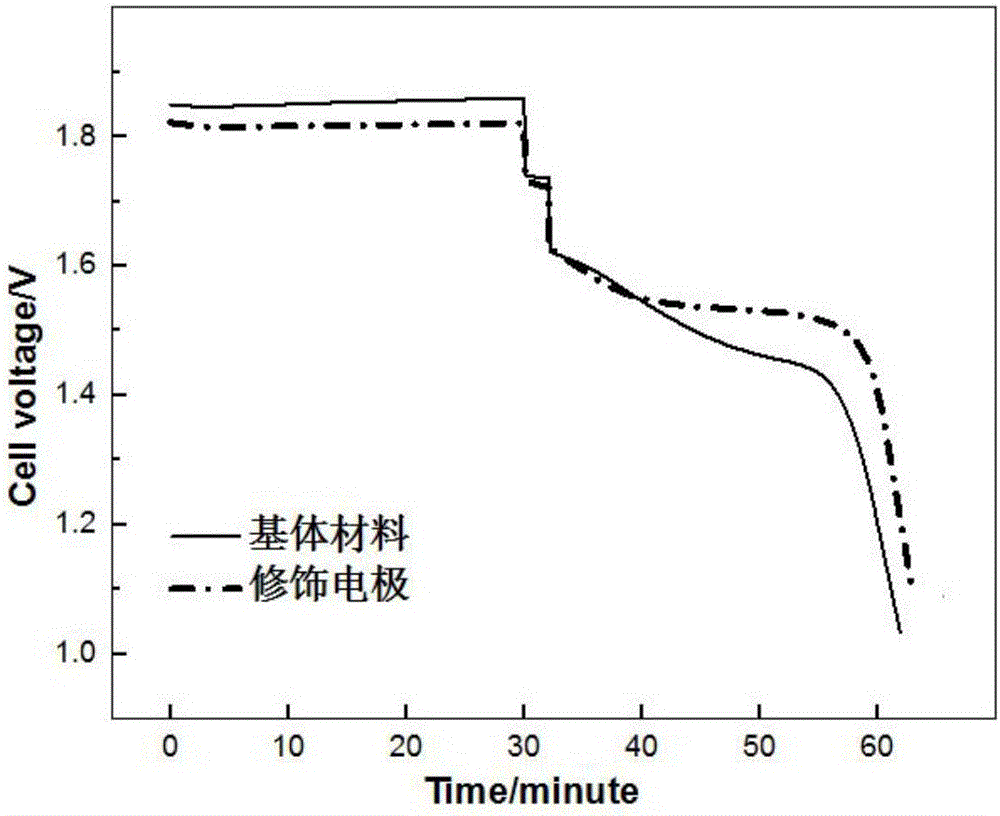
Modified electrode applied to zinc-bromine redox flow battery and preparation method thereof
ActiveCN106159286ASimple structureEasy to prepareCell electrodesRegenerative fuel cellsFiberCarbon felt
The invention belongs to the technical field of surface treatment of electrode materials and particularly relates to a modified electrode applied to a zinc-bromine redox flow battery and a preparation method thereof. A base material of the modified electrode can be a graphite sheet or a carbon felt; noble metal nano-particles, such as noble metal nano-particles including platinum, gold, palladium and iridium, are attached on the surface of the graphite sheet or a fibrous surface of the carbon felt. The preparation method of the modified electrode is characterized by comprising the following steps of: preparing a precursor solution from acids and salts including chloroplatinic acid, chloroauric acid, ammonium chloropalladate or iridium trichloride and the like and ultrasonically vibrating for 2 minutes; then immersing the graphite sheet or the carbon felt into the precursor solution; and adding reducing agents including ascorbic acid or hydrazine hydrate and the like and reducing the noble metal nano-particles to the graphite sheet or the fibrous surface of the carbon felt from the relative precursor solution, so as to finish the preparation of the modified electrode. The invention provides the modified electrode which is simple in structure and the preparation method of the modified electrode is convenient; after the modified electrode is applied to the zinc-bromine redox flow battery, the problem that the activity of the electrode of the zinc-bromine redox flow battery is not enough can be solved.
Owner:UNIV OF SCI & TECH BEIJING
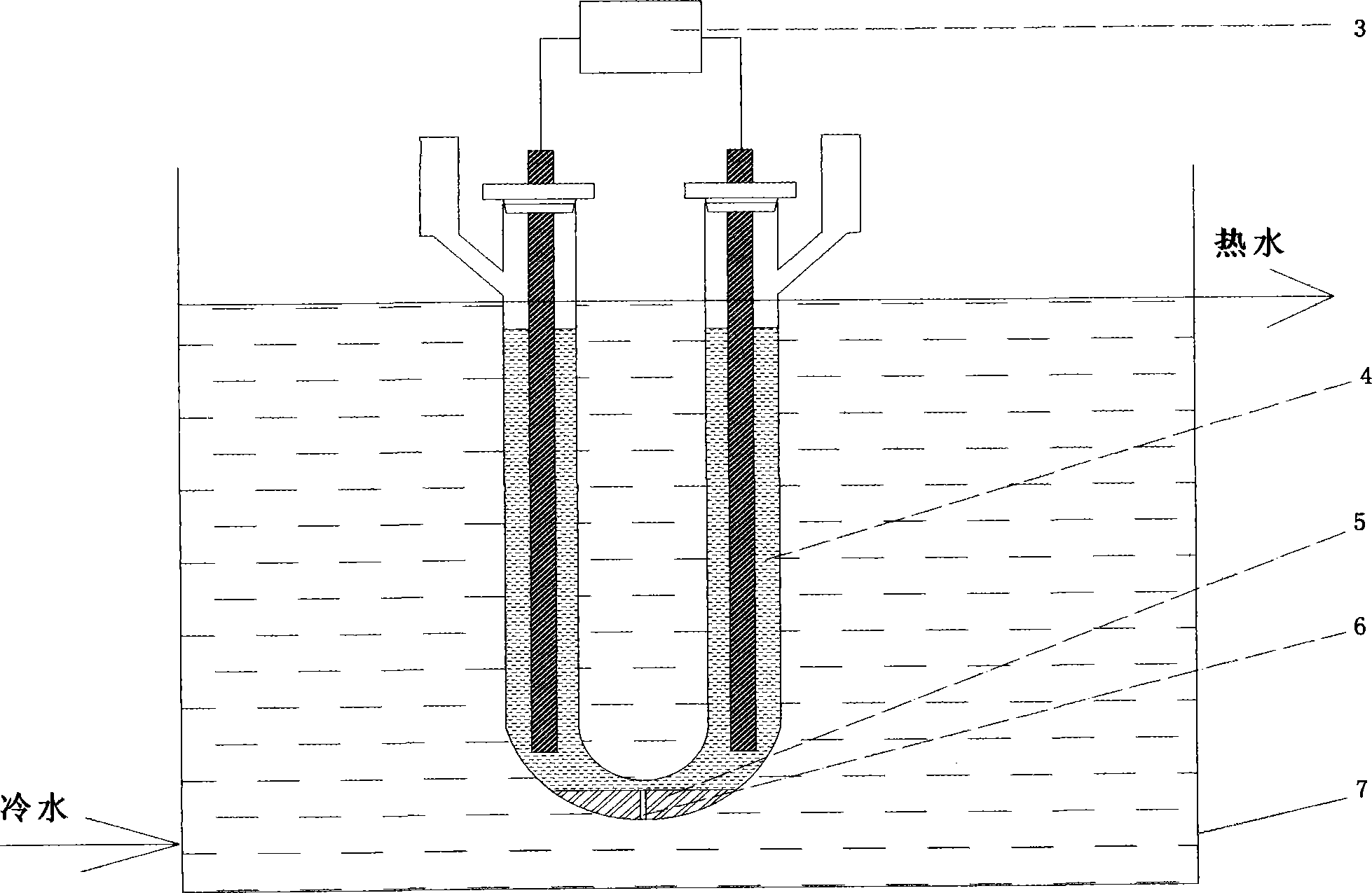
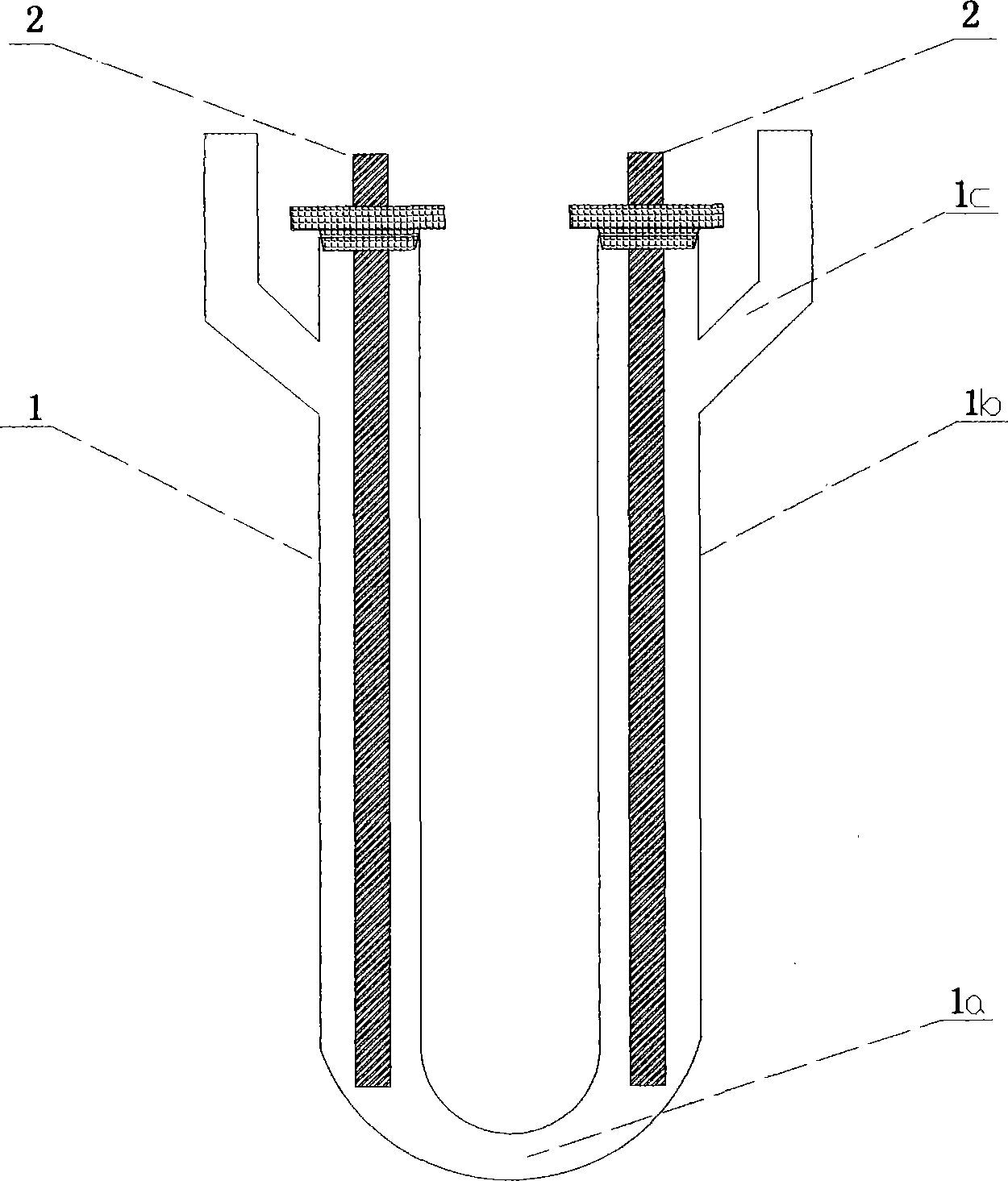
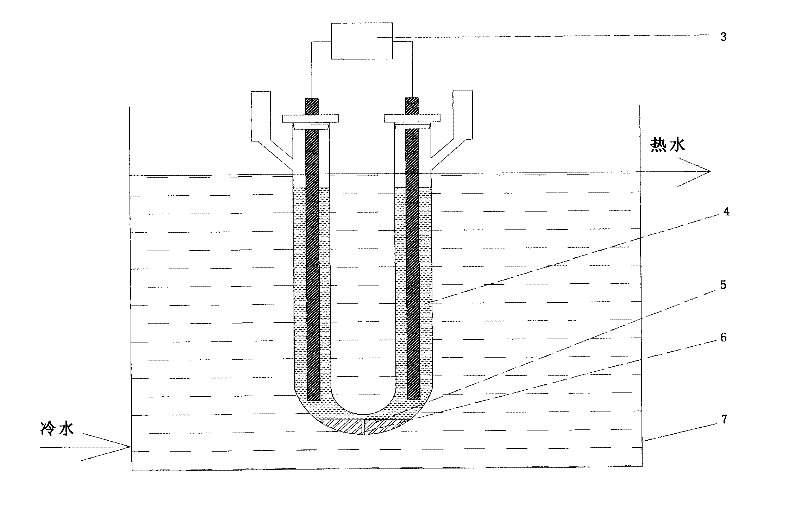
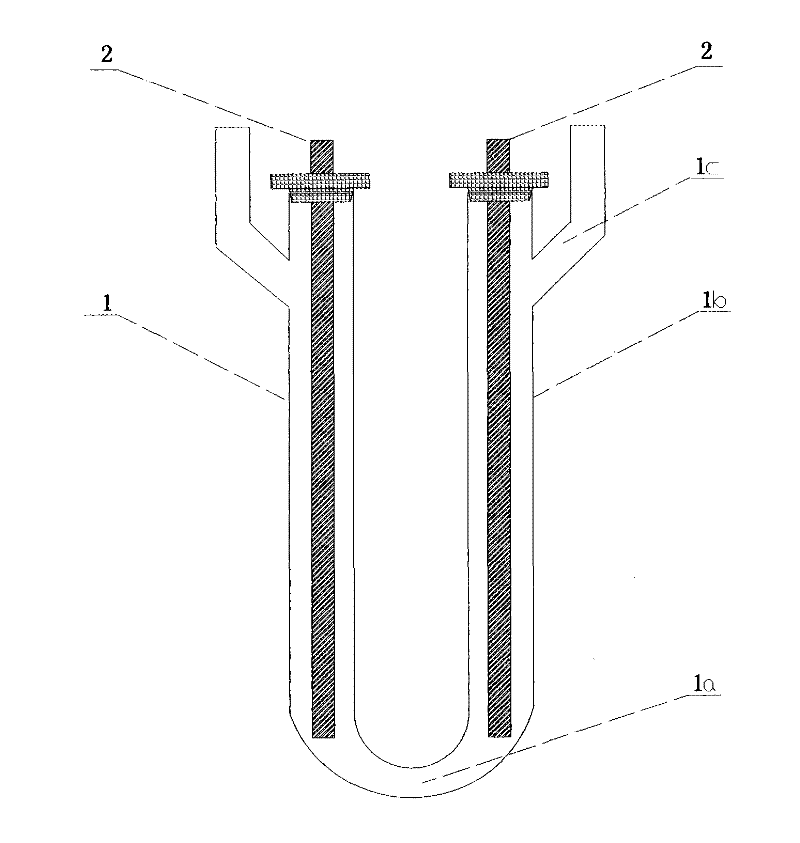
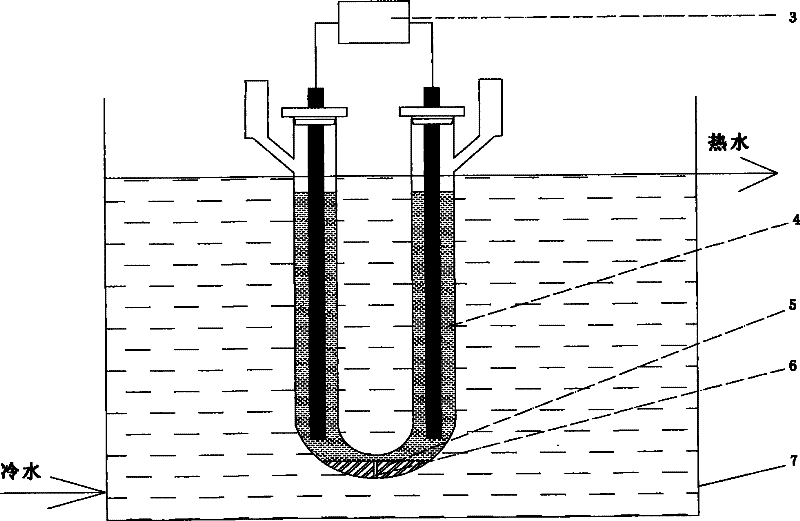
Method for preparing iridous chloride hydrate
ActiveCN102408135AThe dissolution process is simpleHigh purityRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsImpurityRaw material
The invention relates to a method for preparing an iridous chloride hydrate (IrCl3.3H2O). The method comprises the following steps of: adding a hydrochloric acid solution and an iridium powder raw material into a U-shaped electrolytic cell, loading alternating current onto the two ends of an electrode, and directly dissolving the iridium powder into hydrochloric acid to obtain a chloroiridic acid aqueous solution; filtering the chloroiridic acid aqueous solution, and distilling to obtain a chloroiridic acid concentrated solution; and crystallizing the chloroiridic acid concentrated solution in a crystallization furnace to obtain an iridous chloride hydrate. In the preparation method of the iridous chloride hydrate, the iridous powder raw material is directly dissolved into the hydrochloric acid without adding any other reagent, so that the production cost is lowered, and a product is crystalized into the iridous chloride hydrate which is easy to carry, has stable iridium concentration, and is convenient to weigh and use; and when a high-purify iridous powder raw material is adopted, the impurity interference can be prevented, and a high-purity iridous chloride hydrate is prepared.
Owner:CHINA PETROLEUM & CHEM CORP +1
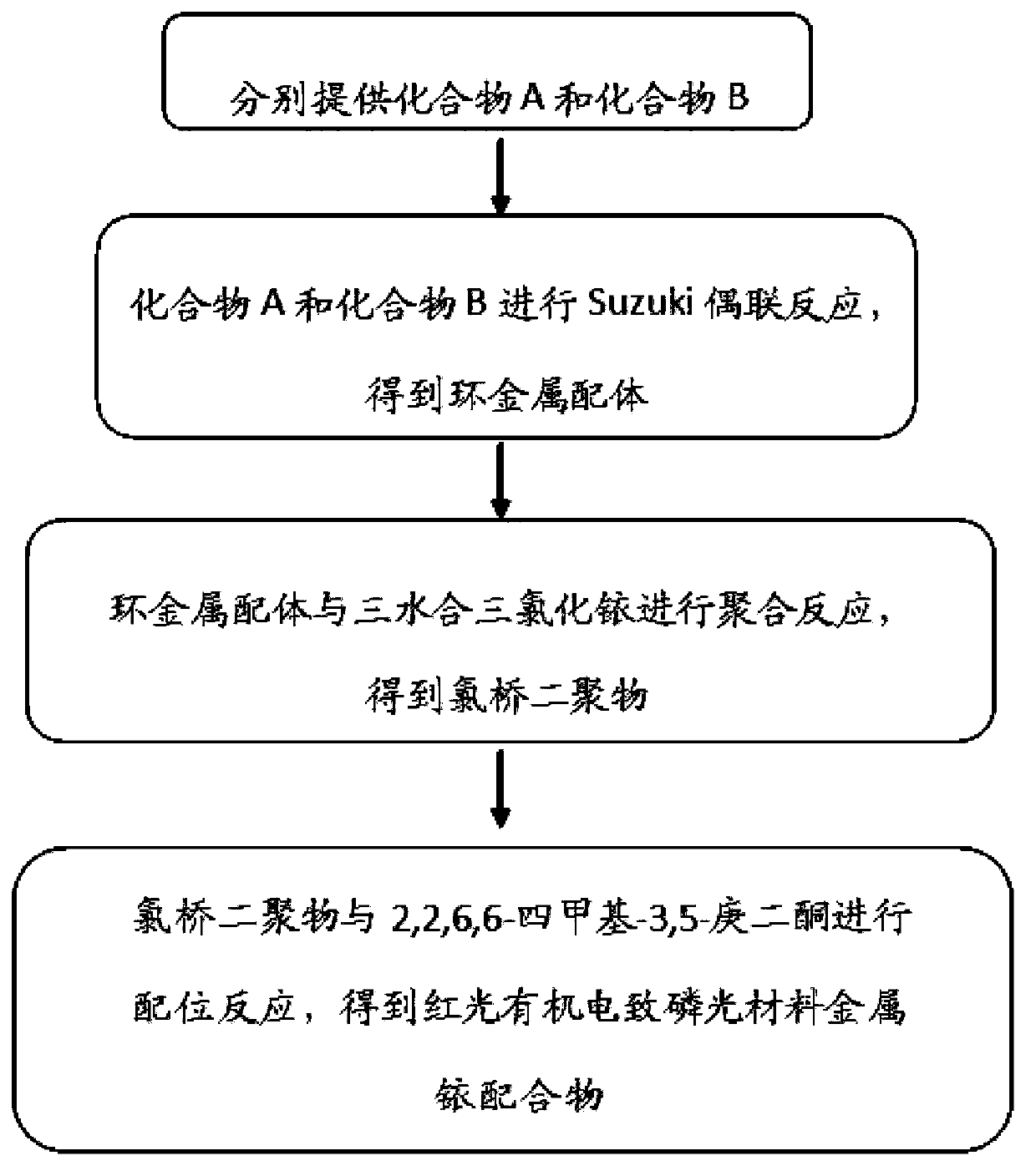
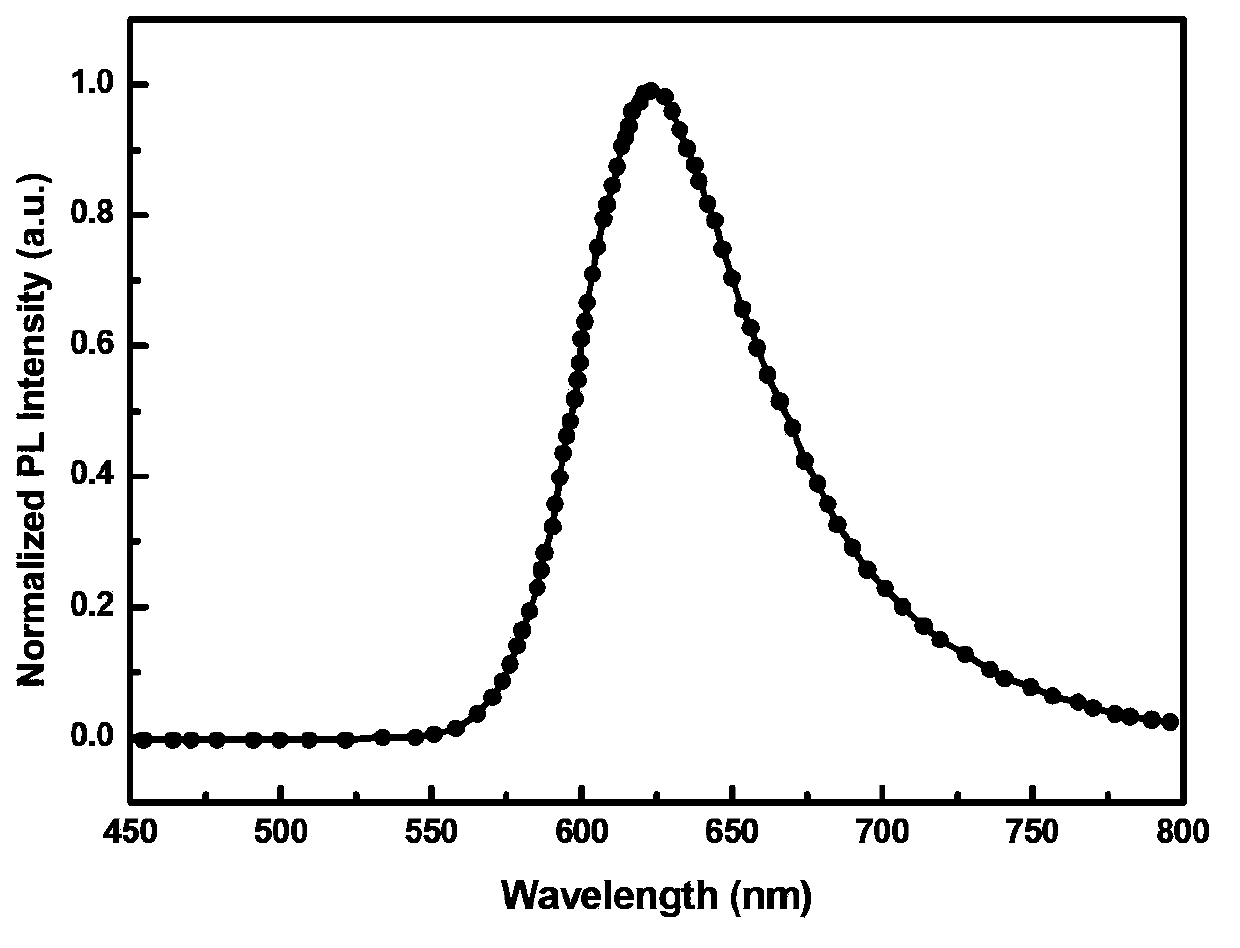
Red light-emitting organic electrophosphorescent material iridium complex, its preparation method and organic electroluminescent device
InactiveCN103965253AIncreased steric hindranceReduce direct effectGroup 8/9/10/18 element organic compoundsSolid-state devicesBiopolymerDirect effects
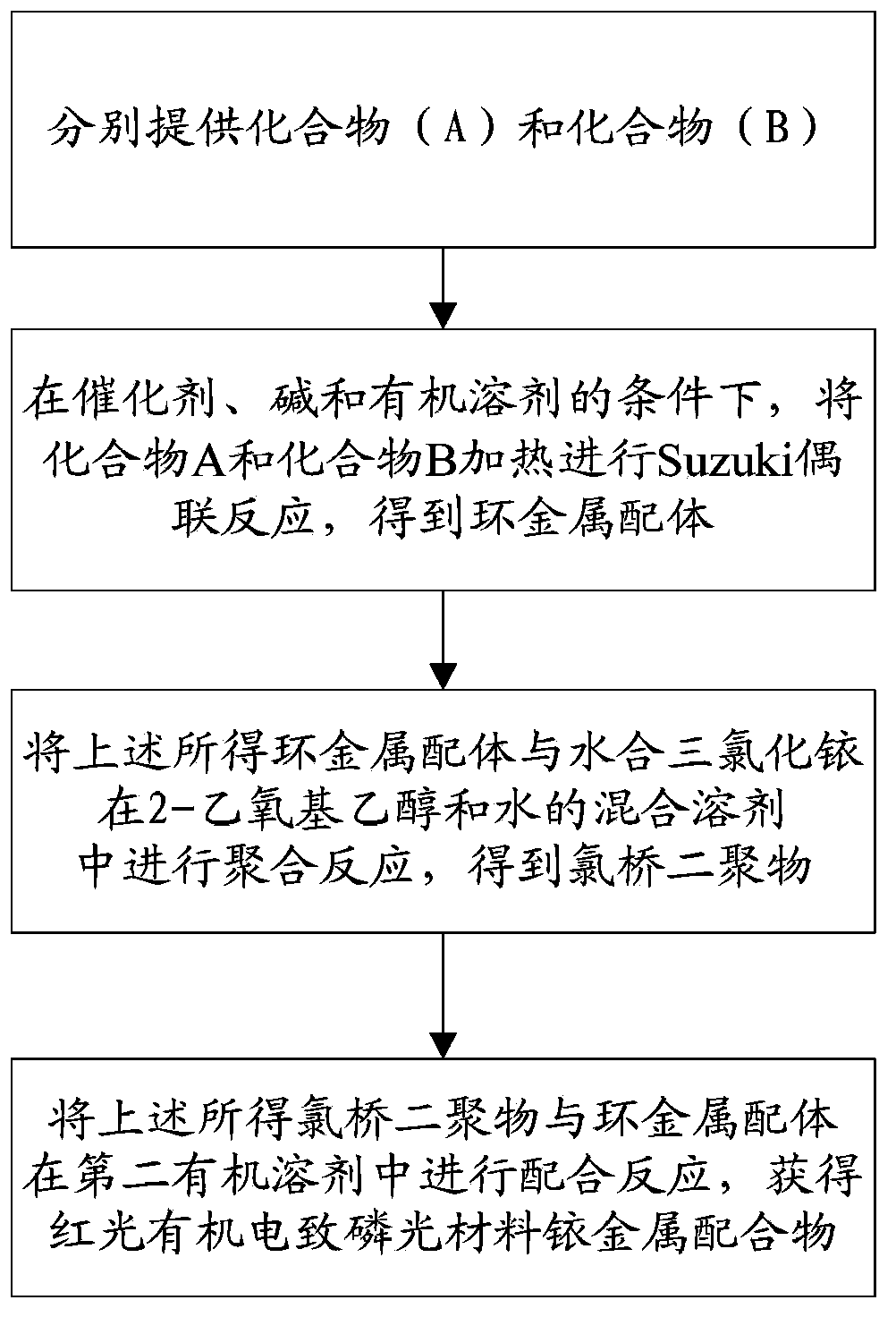
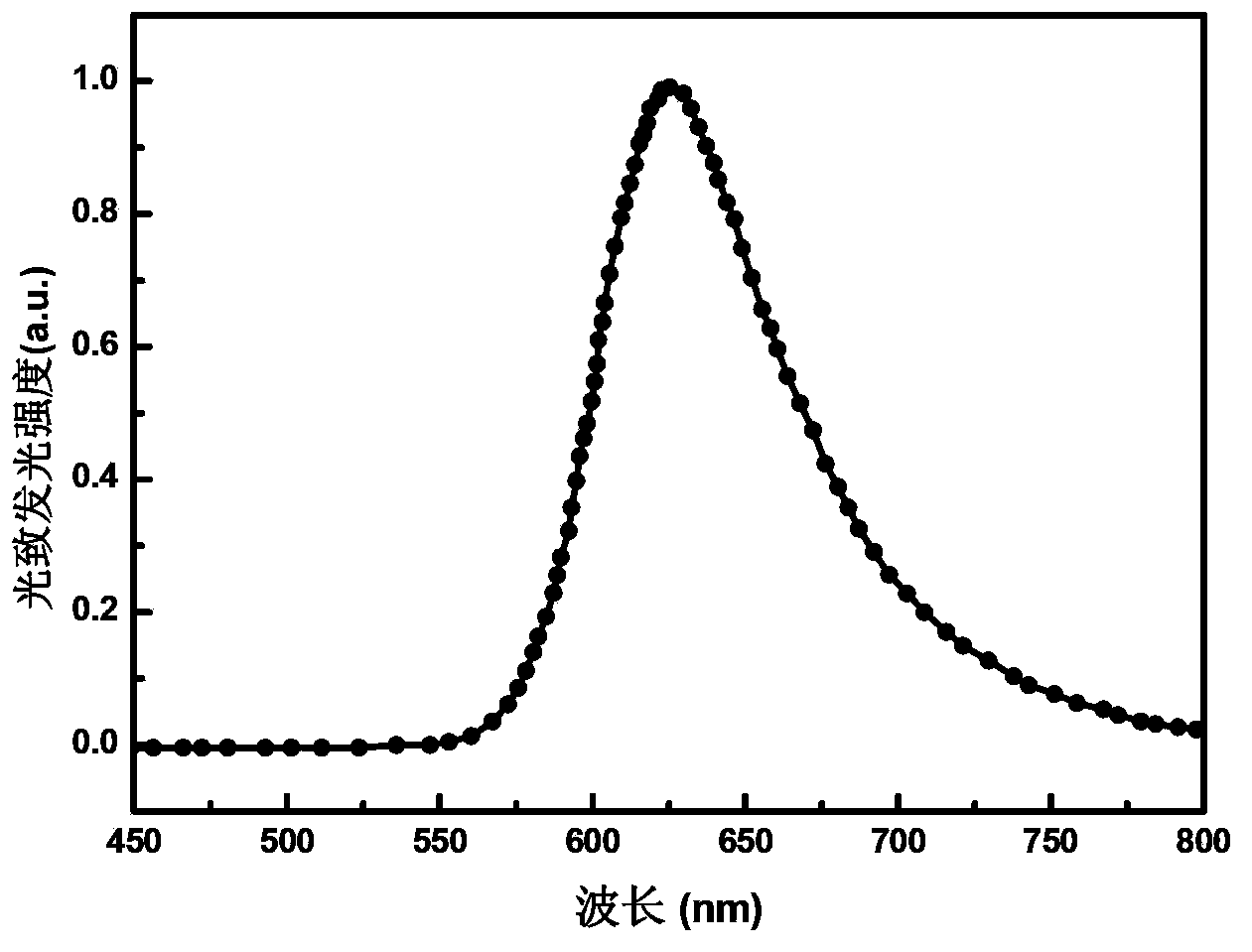
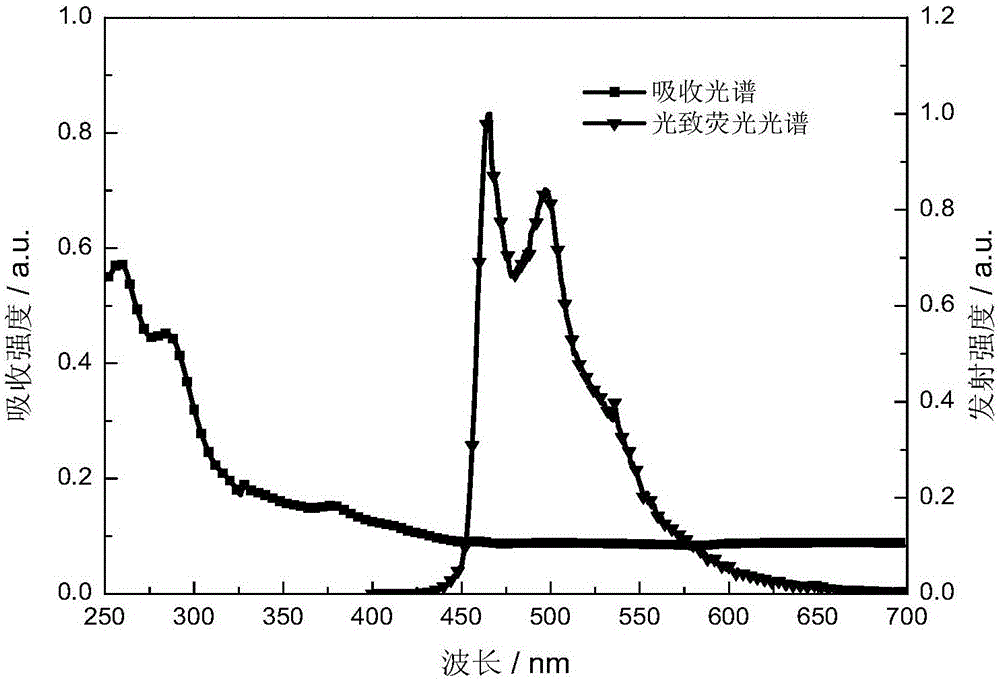
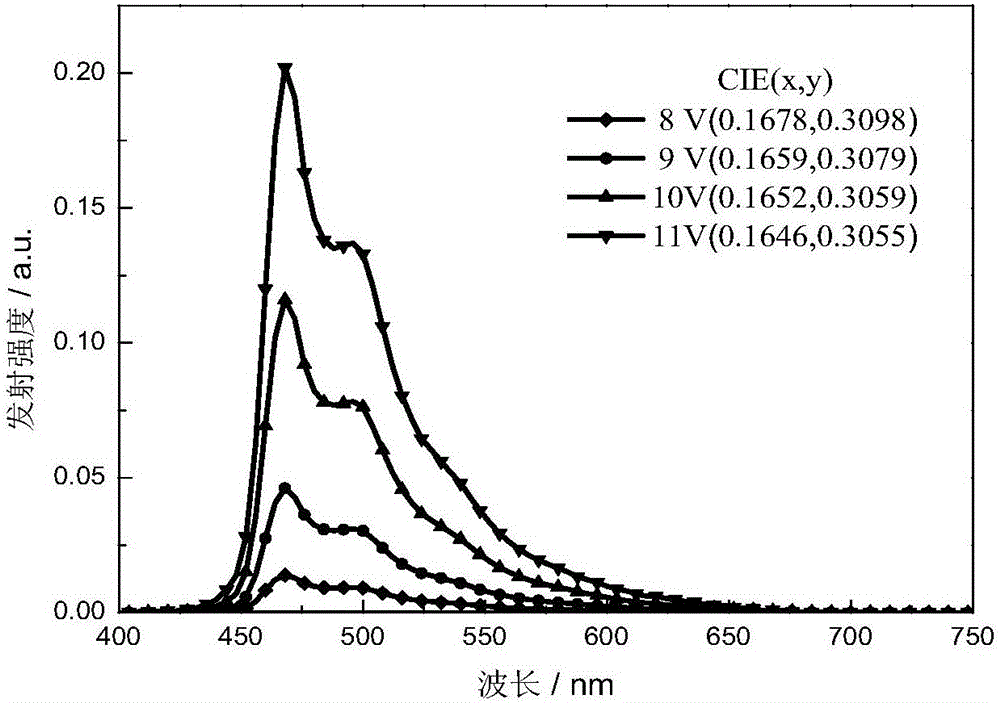
The invention discloses a red light-emitting organic electrophosphorescent material iridium complex having a structure shown in the formula (P), and in the formula, R represents methyl. The preparation method comprises the following steps that a ring metal ligand is prepared by a Suzuki coupling reaction, the ring metal ligand and an iridium trichloride trihydrate undergo a polymerization reaction in a 2-ethoxyethanol-water mixed solvent to produce a chlorine-bridged biopolymer, and the chlorine-bridged biopolymer and 2,2,6,6-tetramethyl-3,5-heptanedione undergo a coordination reaction to produce the red light-emitting organic electrophosphorescent material iridium complex. The invention also provides the organic electroluminescent device containing the iridium complex. Through use of 2,2,6,6-tetramethyl-3,5-heptanedione as an assistant ligand in the ring metal ligand, metal iridium complex steric-hinerance effect is improved so that direct effects between metal atoms are reduced, triplet exciton self-quenching phenomenon is reduced and luminescence efficiency of the organic electroluminescent device is improved.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Iridium-based nanowire synthesis method
ActiveCN112475314AReduce wasteLarge reaction surfaceMaterial nanotechnologyTransportation and packagingNanowireSodium iodide
The invention discloses an iridium-based nanowire synthesis method. The method comprises the following steps: (1) selecting a dimethylformamide solution, iridium trichloride, a metal source, polyvinylpyrrolidone and sodium iodide as raw materials, placing the dimethylformamide solution, the iridium trichloride, the metal source and the polyvinylpyrrolidone into a same reactor, and uniformly mixingto obtain a solution 1; (2) adding the sodium iodide into ultrapure water to obtain a solution II; (3) mixing the solution I and the solution II, and carrying out uniform ultrasonic dispersion to obtain a mixed reaction solution; (4) heating the mixed reaction solution to T1 in a gradient manner, keeping the temperature unchanged, and reacting for a period of time t2 to obtain a black solution; and stopping the reaction, and after the whole system is cooled, centrifuging and washing the product to obtain an iridium-based nanowire. The iridium-based nanowire synthesized through the method is good in performance, high efficiency and high stability of oxygen evolution under the acidic condition can be achieved, the catalytic energy barrier of oxygen evolution semi-reaction is reduced, and the iridium-based nanowire has high potential practical value.
Owner:QINGDAO UNIV
Nickel iridium-oxide composite catalyst and preparation method thereof
InactiveCN104492457AHigh catalytic activityImprove stabilityCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsNickel saltElectrochemistry
The invention relates to a nickel iridium-oxide composite catalyst and a preparation method thereof. The invention is characterized in that a stainless steel screen framework with the mesh diameter of 1-100 mu m can be subjected to electrochemical codeposition to obtain the compact nickel iridium-oxide composite catalyst with uniform component distribution, fine crystal grain, carbon deposition resistance, high catalytic activity and enduring stability. The oxide in the composite catalyst is composed of one or more of SiO2, ZrO2, CeO2, La2O3, Al2O3, TiO2 and the like, and the iridium content in the catalyst is 0.01-20 at%. The preparation method of the composite catalyst comprises the following steps: dissolving 0.001-1.0 mol / L nickel salt, 0.005-0.100 mol / L iridium salt crystalline water iridous chloride, 0.1-1.0 mol / L complex citric acid, 0.1-100 g / L nano oxide particle and 0.0001-0.005 mol / L additive in deionized water according to certain molar concentration, and carrying out nickel iridium-oxide composite catalyst electrochemical codeposition on the pretreated workpiece electrode surface under the auxiliary action of ultrasonic to obtain the nickel iridium-oxide composite catalyst.
Owner:CHANGZHOU UNIV
Preparation method and application of high-efficiency hydrogen evolution catalyst Ir@NBD-C
ActiveCN111558390AHigh HER activityImprove electrochemical performancePhysical/chemical process catalystsElectrodesHydration reactionPtru catalyst
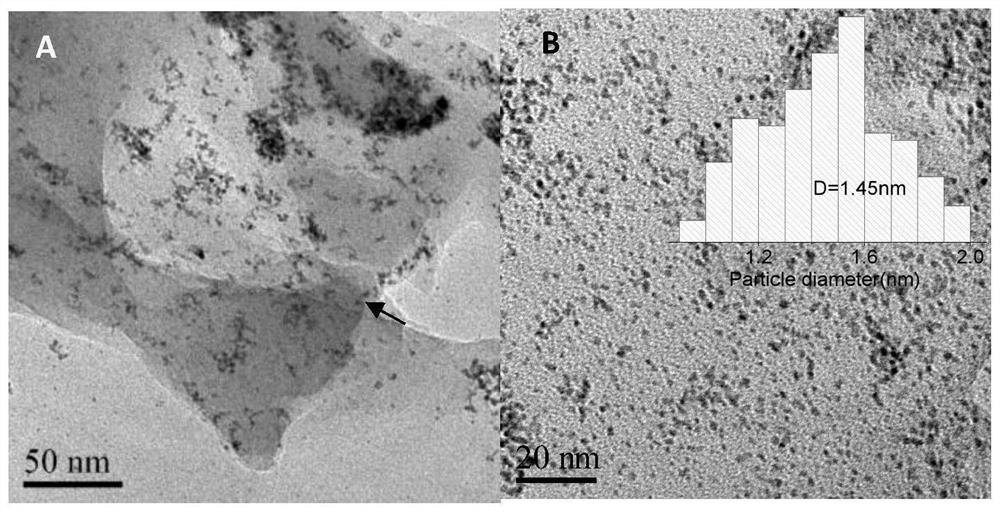
The invention relates to a preparation method of a high-efficiency hydrogen evolution (HER) catalyst Ir@NBD-C. The preparation method comprises the following steps: uniformly mixing a defective carbonsubstrate with deionized water; then adding iridium trichloride hydrate, melamine and boric acid, uniformly mixing, and drying to obtain a powder sample; and calcining for 1-2 hours at 600 + / - 50 DEGC in an inert atmosphere to obtain the hydrogen evolution catalyst Ir@NBD-C-600. The growth speed of particles is reduced by utilizing the binding effect of nitrogen and boron on metal single atoms and metal clusters, the diameter of noble metal nanoparticles is reduced to 2 nm or less, and the noble metal nanoparticles are uniformly distributed on the carbon substrate. The particle diameter is reduced, so that the catalyst has a higher specific surface area; and the catalyst can expose more active surfaces beneficial to catalysis. Further, the overpotential of the catalyst under a certain current density in the HER process is obviously reduced; and due to the binding effect of nitrogen and boron, the metal nanoparticles keep good stability in the circulation process.
Owner:ZHENGZHOU UNIV
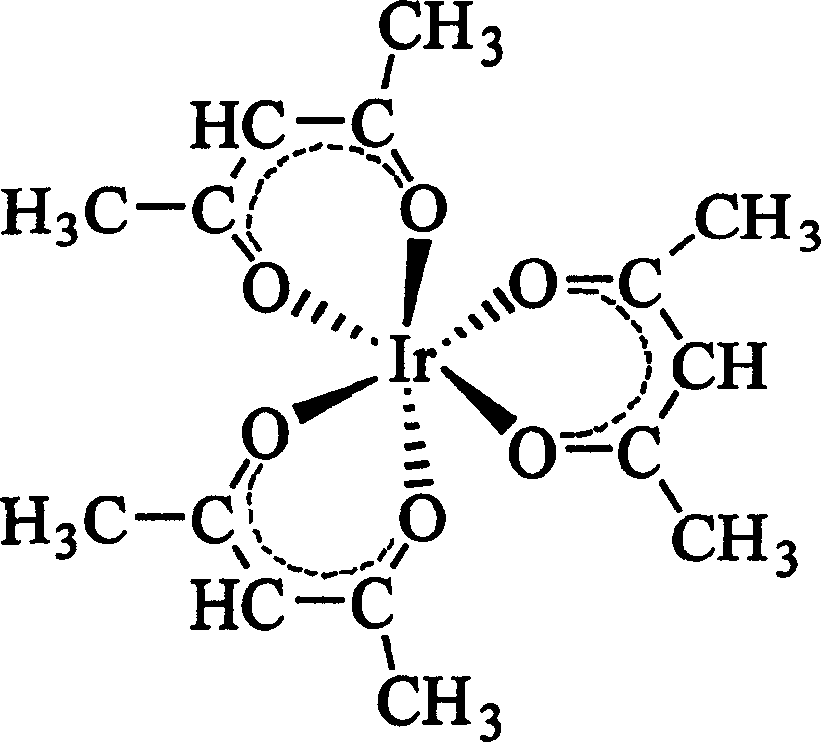
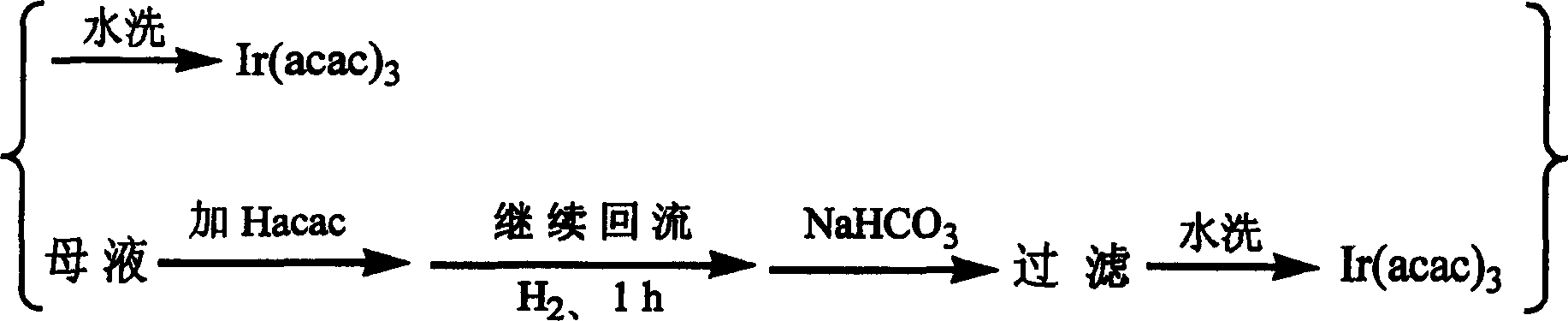
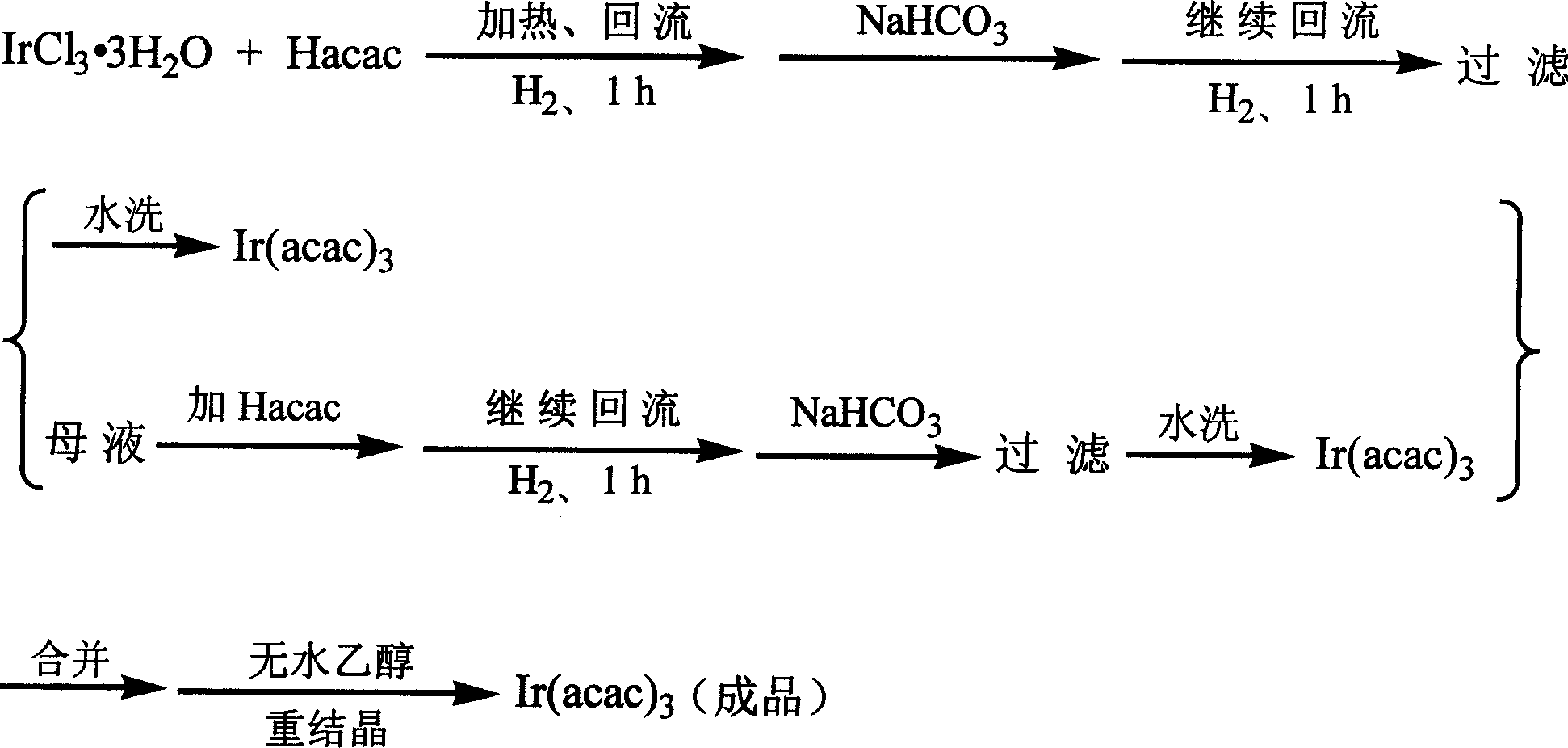
Method for synthesizing iridium (III) triacetylacetonate
InactiveCN1546499ABreak the situation of dependence on importsHigh yieldGroup 8/9/10/18 element organic compoundsSodium bicarbonateHydrogen
The invention relates to a method for synthesizing iridium (III) acetylacetonate which comprises, dissolving iridium (III) chloride trihydrated into thermal distillation water, charing acetylacetone when agitating and letting in hydrogen gas, backflow, dropping saturated sodium bicarbonate solution, backflow, stopping hydrogen gas, filtering to obtain the end product.
Owner:KUNMING INST OF PRECIOUS METALS
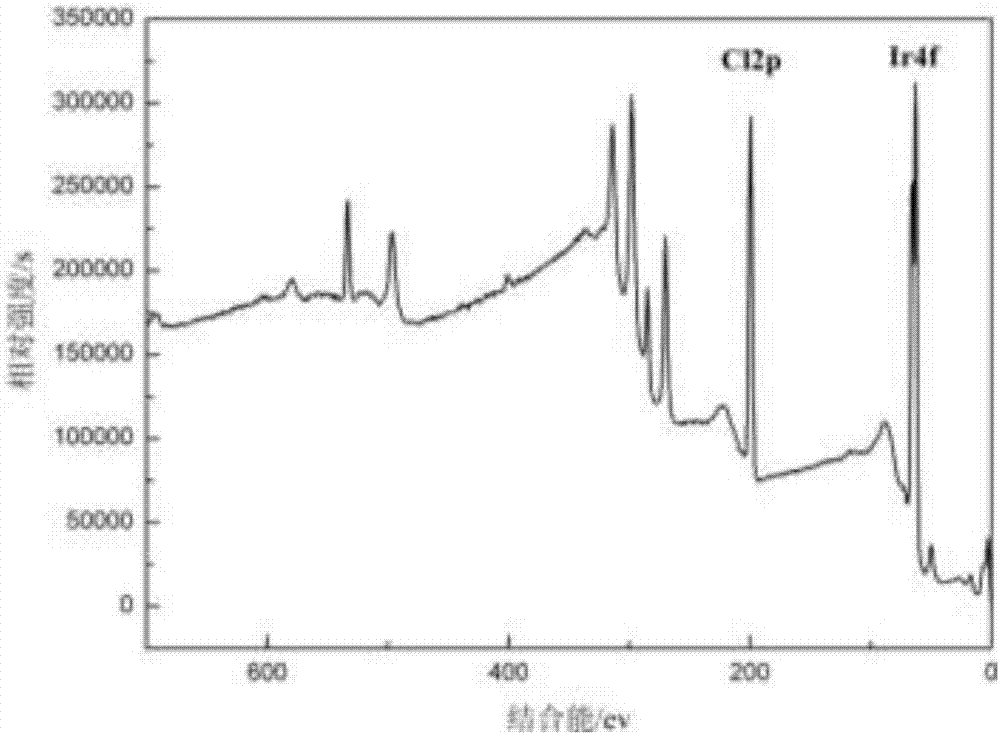
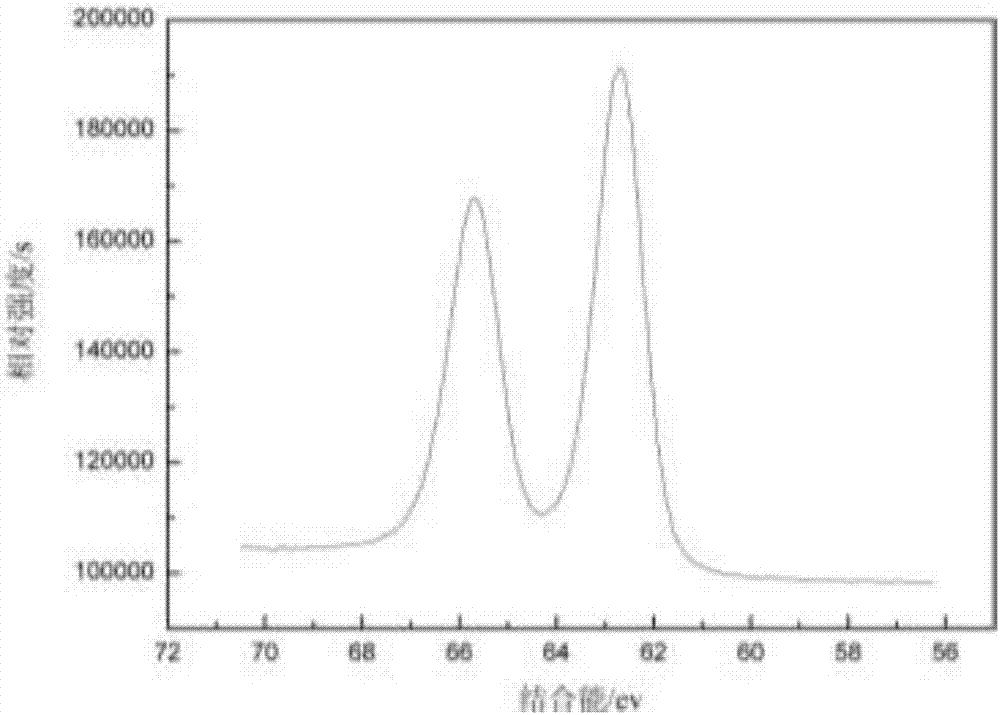
Preparation method of high-purity iridium trichloride
PendingCN114105229AIncrease productivityHigh yieldRuthenium/rhodium/palladium/osmium/iridium/platinum halidesProcess efficiency improvementNitrosoWater chlorination
The invention discloses a method for preparing high-purity iridium trichloride. Iridium powder is used as a raw material to be subjected to alkali fusion under the mixing action of sodium peroxide, sodium hydroxide and sodium carbonate; dissolving the alkali fusion solid with aqua regia, adding ammonium chloride into the filtered solution to form precipitated ammonium chloroiridate, reacting the ammonium chloroiridate with hydrazine hydrochloride to form ammonium chloroiridate, further removing impurities by sodium sulfide and ammonium sulfide, filtering, oxidizing the filtrate by hydrochloric acid / hydrogen peroxide, and precipitating by an ammonium chloride solution to obtain ammonium chloroiridate with higher purity; the preparation method comprises the following steps: removing nitrate from ammonium chloroiridate by using an aqua regia solution, adding sodium nitrite / potassium chloride into the obtained chloroiridate solution, boiling to obtain a potassium hexanitroso iridate solid, filtering, adding hydrochloric acid into the solid, boiling, dissolving, removing nitrate, adding a proper amount of hydrazine hydrochloride, and carrying out rotary evaporation and vacuum calcination on the solution to obtain an iridium trichloride solid with the purity of more than 99.995%.
Owner:英特派铂业股份有限公司
Controlled reduction preparation method of iridium trichloride
ActiveCN106854001AAvoid introducingHigh purityRuthenium/rhodium/palladium/osmium/iridium/platinum halidesRefluxIridium chloride
Belonging to the technical field of reduction, the invention discloses a controlled reduction preparation method of iridium trichloride. The method includes: adding a quadrivalent iridium chloride and a reducing agent in a three-mouth flask according to certain proportion, under a magnetic stirring condition, and conducting constant temperature reduction until quadrivalent iridium in the solution is completely converted to trivalence. A condensation reflux device is used in the reaction process to reduce the volatilization of the reducing agent and control the reaction system stable. The method has the advantages of simple operation, short process, fully controllable reduction process and stable content, can guarantee that quadrivalent iridium can be completely reduced to trivalence, and the prepared iridium trichloride product has high purity.
Owner:GRIKIN ADVANCED MATERIALS
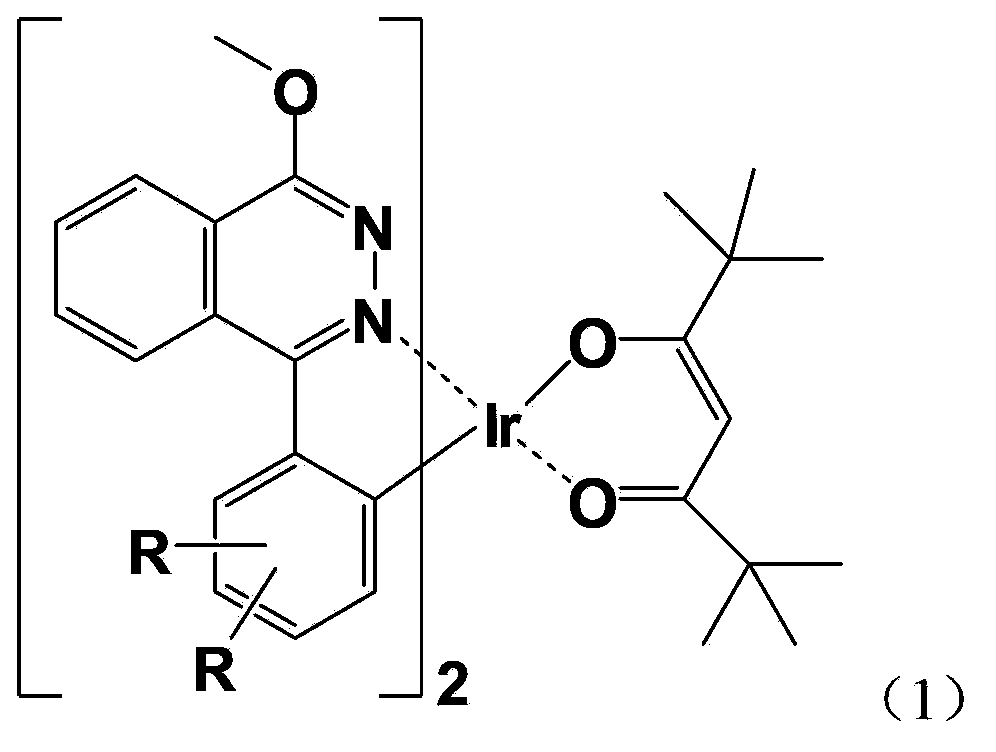
Red organic electrophosphorescent material iridium metal complex, preparation method thereof, and organic electroluminescent device
InactiveCN104140447AGood energy transfer efficiencyAppropriate red light emission wavelengthGroup 8/9/10/18 element organic compoundsSolid-state devicesSolventCoordination complex
The invention provides a red organic electrophosphorescent material iridium metal complex with the structure represented by formula (1). In the formula (1), R is a hydrogen atom or a C1-C4 straight chain or alkoxy group. The red organic electrophosphorescent material iridium metal complex is prepared through the following steps: carrying out a Suzuki coupling reaction on an iridium metal complex to prepare a cyclomedtalating ligand, carrying out a polymerization reaction on the cyclomedtalating ligand and chromium trichloride hexahydrate in a 2-ethoxyethanol and water mixed solvent to obtain a chlorendic dimer, and carrying out a complex reaction on the chlorendic dimer and the cyclomedtalating ligand to obtain the red organic electrophosphorescent material iridium metal complex represented by formula (1). The above material has good energy transmission efficiency and appropriate red light emitting wavelength, and can be widely used to make red or white phosphorescent electroluminescent devices in order to reduce the power consumption of the devices, improve the performances of the devices and prolong the life of the devices.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
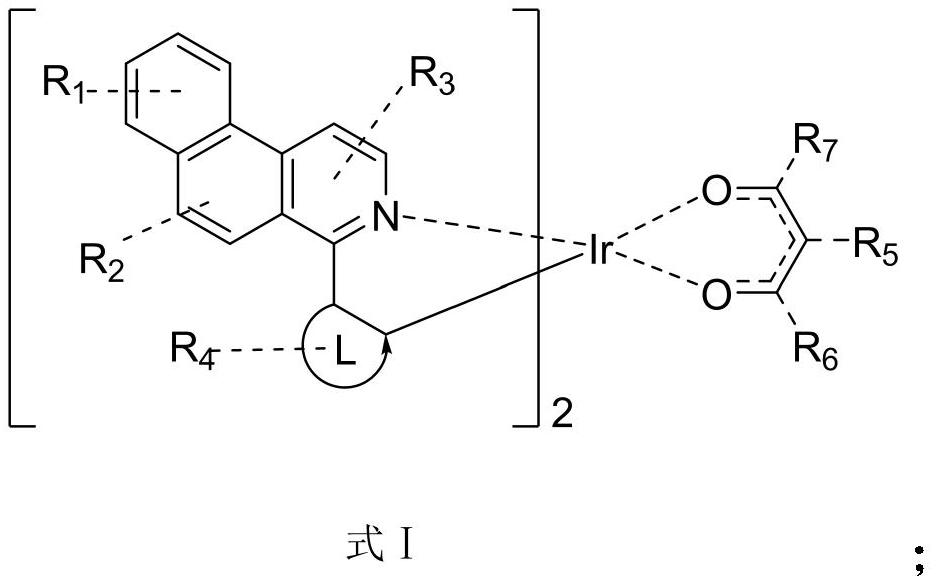
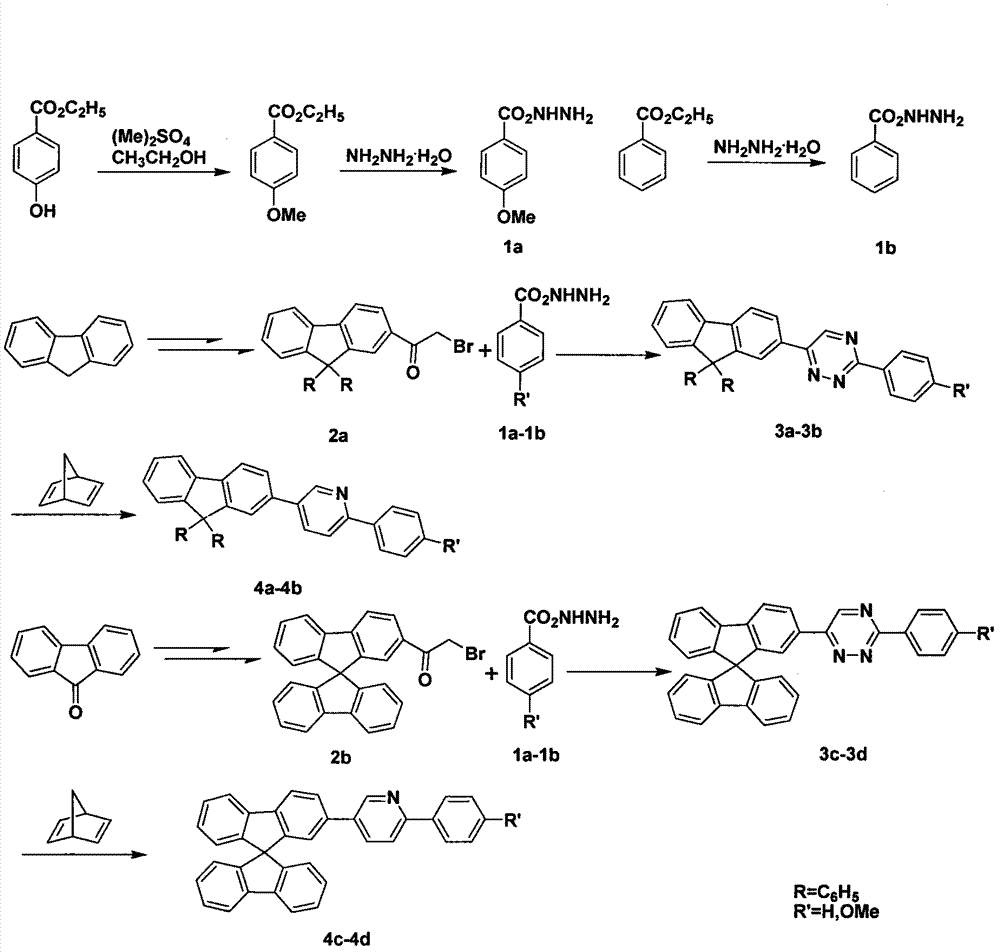
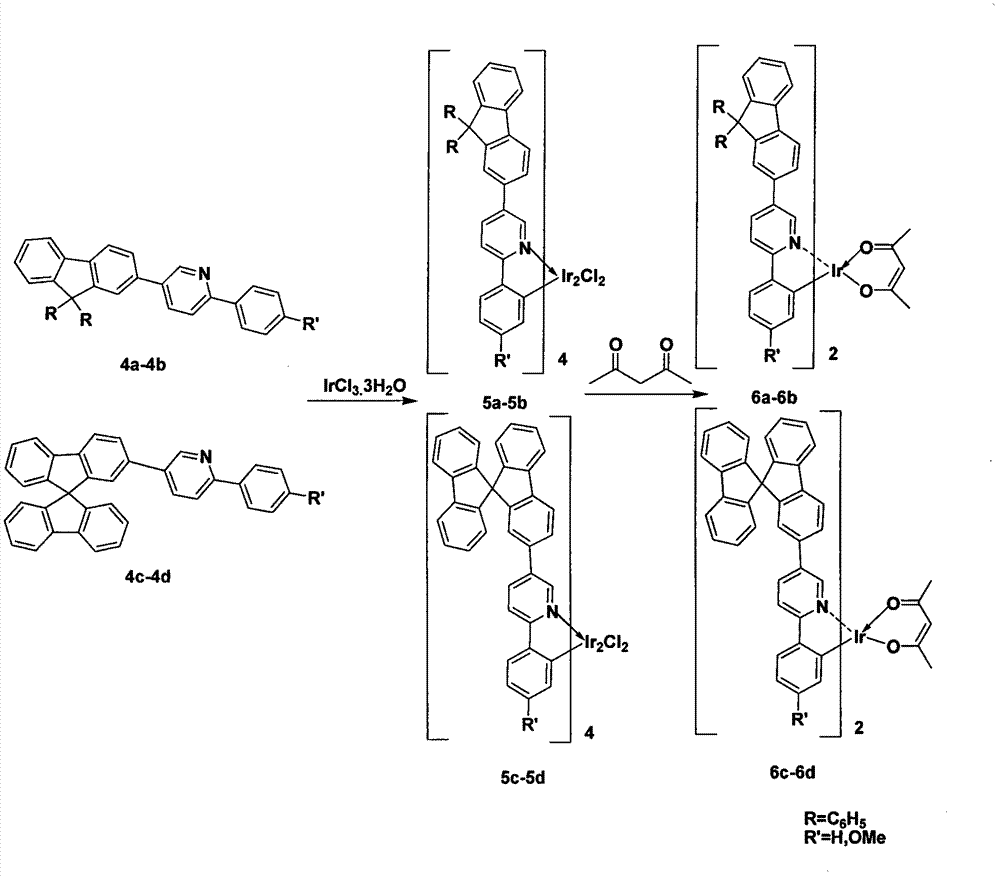
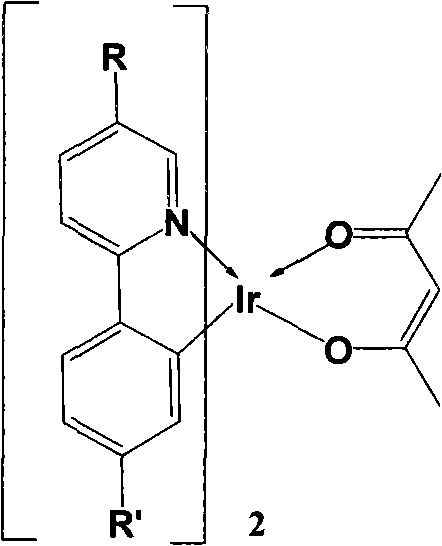
Multi-substituted phenylpyridine iridium (III) complex and preparation method and application thereof
InactiveCN106317123AImprove quantum efficiencyDual emission with blue lightIndium organic compoundsLuminescent compositionsStructural formulaIridium chloride
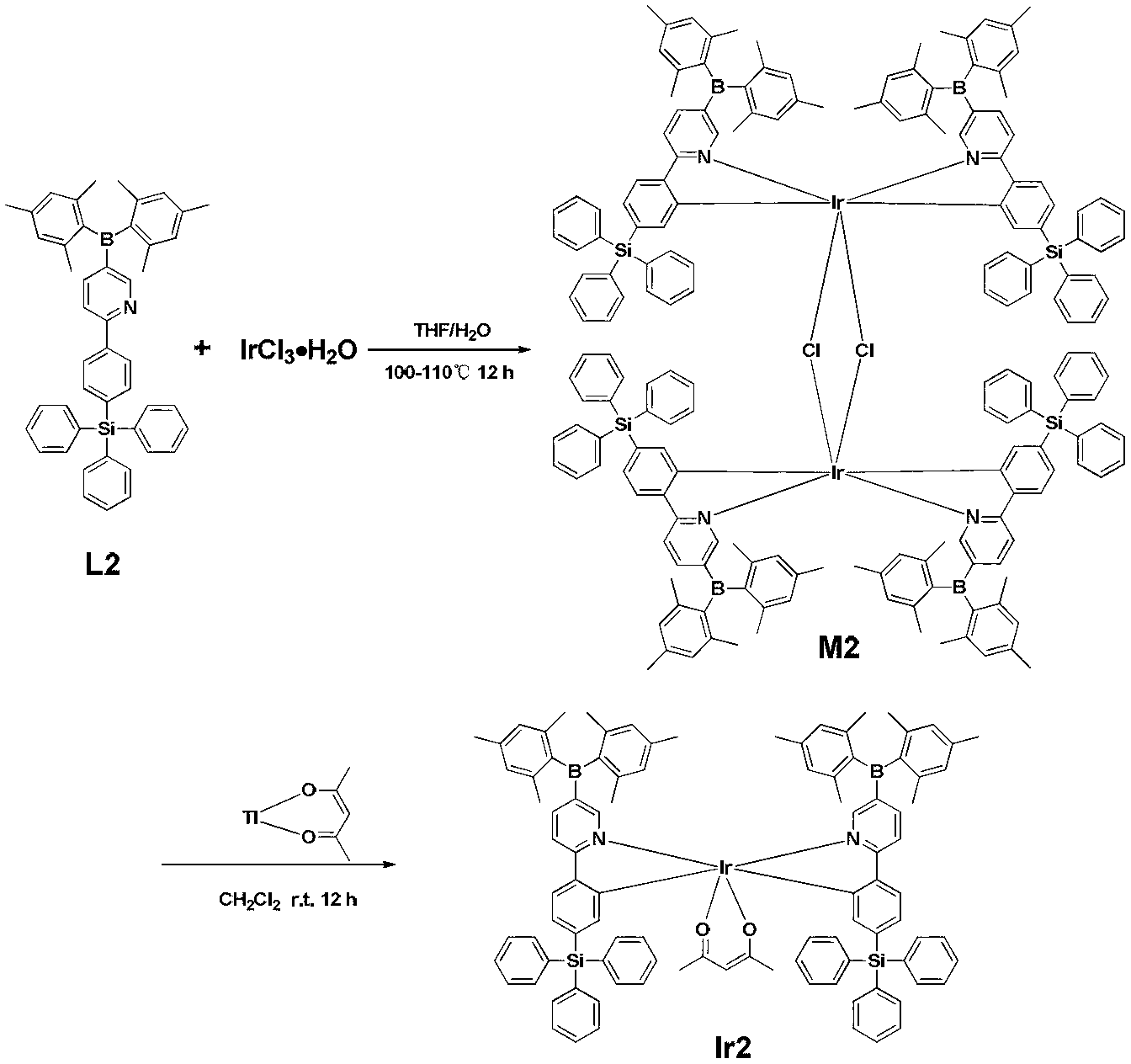
The invention provides a multi-substituted phenylpyridine iridium (III) complex and a preparation method and application thereof. The structural formula of the complex is as shown in the formula I or II. Please see the formula in the description. R1, R2, R3, R4 and R5 are independently hydrogen, fluorine, methyl or trifluoromethyl. The preparation method includes the steps that multi-substituted phenylpyridine reacts with iridium chloride trihydrate to obtain iridium (III) chloro-bridged dimer; then the iridium (III) chloro-bridged dimmer reacts with 2-pyridinecarboxylic acid or 3-trifluoromethyl-5-pyridyl triazole, and the multi-substituted phenylpyridine iridium (III) complex can be obtained. The multi-substituted phenylpyridine iridium (III) complex has the advantages of blue light dual emission, extremely high quantum efficiency and the like, can serve as an electroluminescent phosphorescent material and a phosphorescent doping material to be used in an organic electroluminescent device, can achieve blue light emission, and can be doped with yellow-orange light to achieve white light emission.
Owner:NANJING UNIV OF POSTS & TELECOMM
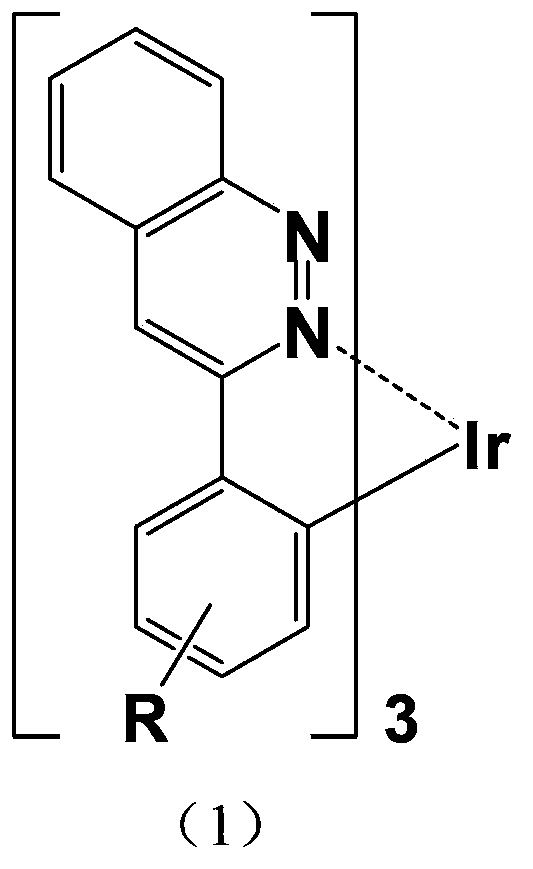
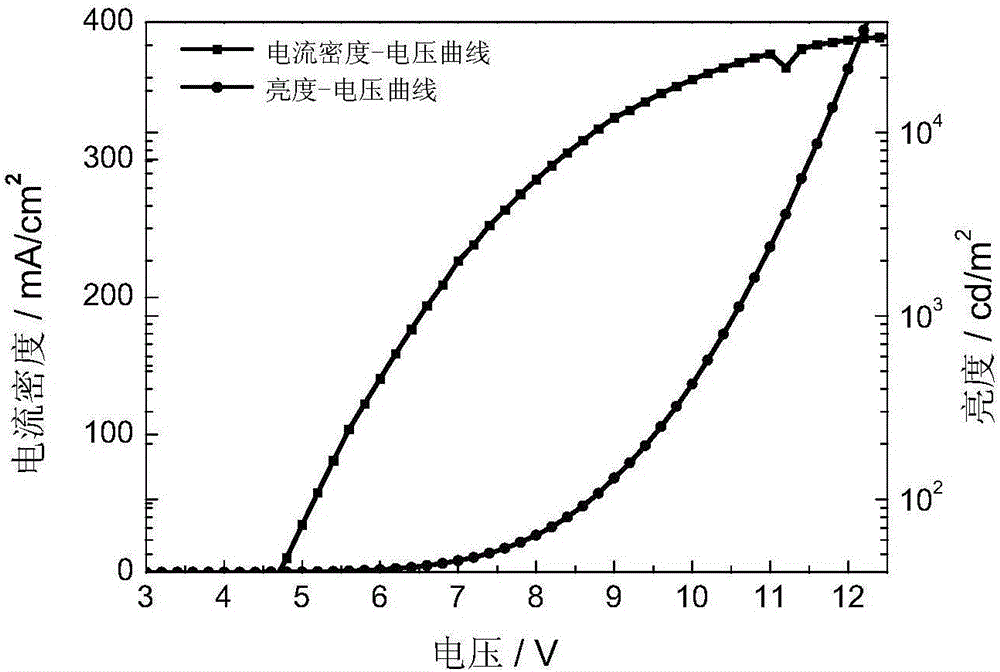
4,7-diarylthieno[2, 3-d] pyridazine cyclometalated iridium complex and preparation method thereof
ActiveCN111377977AEasy to manufactureLow costIndium organic compoundsLuminescent compositionsPhenylboronic acidAryl
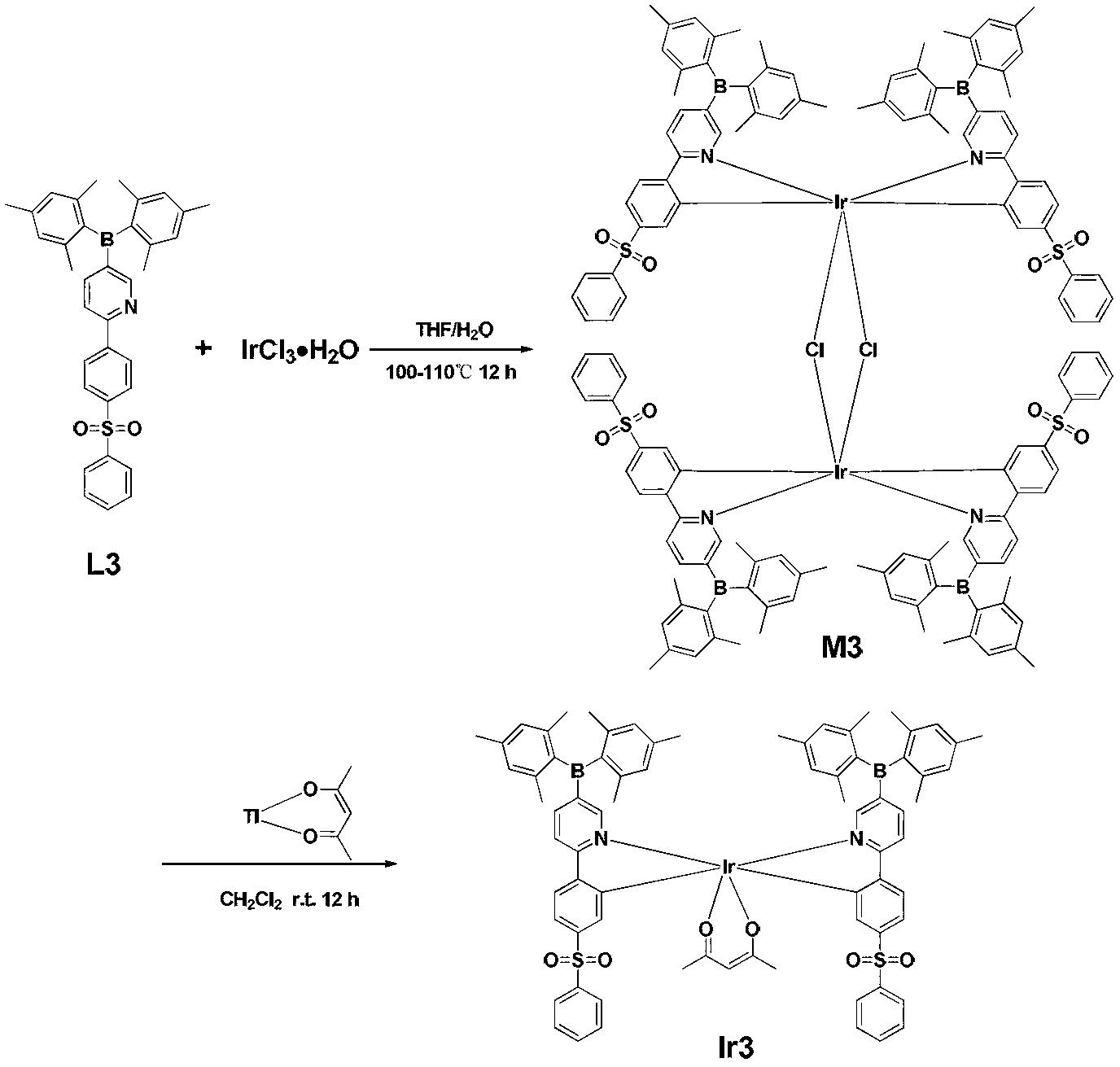
The invention discloses a 4,7-diarylthieno[2, 3-d] pyridazine cyclometalated iridium complex and a preparation method thereof, which belongs to the field of organic photoelectric materials. Accordingto the 4,7-diarylthieno[2,3-d] pyridazine cyclometalated iridium complex disclosed by the invention, the problems that an existing cyclometalated iridium complex is very sensitive to oxygen and a pyridazine iridium complex is not high in quantum efficiency are solved; the preparation method comprises the following steps: substituted phenylboronic acid or boric acid ester is added into chlorothieno[2, 3-d]pyridazine, meanwhile, 4,7-diarylthieno[2,3-d] pyridazine ligands are obtained through a reaction for a period of time in the presence of alkali, a catalyst and a solvent, and the molar ratioof chlorine to substituted phenylboronic acid or boric acid ester in chlorothieno [2,3-d] pyridazine is 1: 1; and the obtained 4, 7-diarylthieno [2,3-d] pyridazine ligand is mixed with iridium trichloride and a solvent, and a reaction is carried out for a period of time to obtain the thieno[2,3-d] pyridazine cyclometalated iridium complex.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Preparation method of vinyl ether compound
InactiveCN102942458AFew synthetic reportsGood effectEther preparation by ester reactionsVinyl etherDistillation
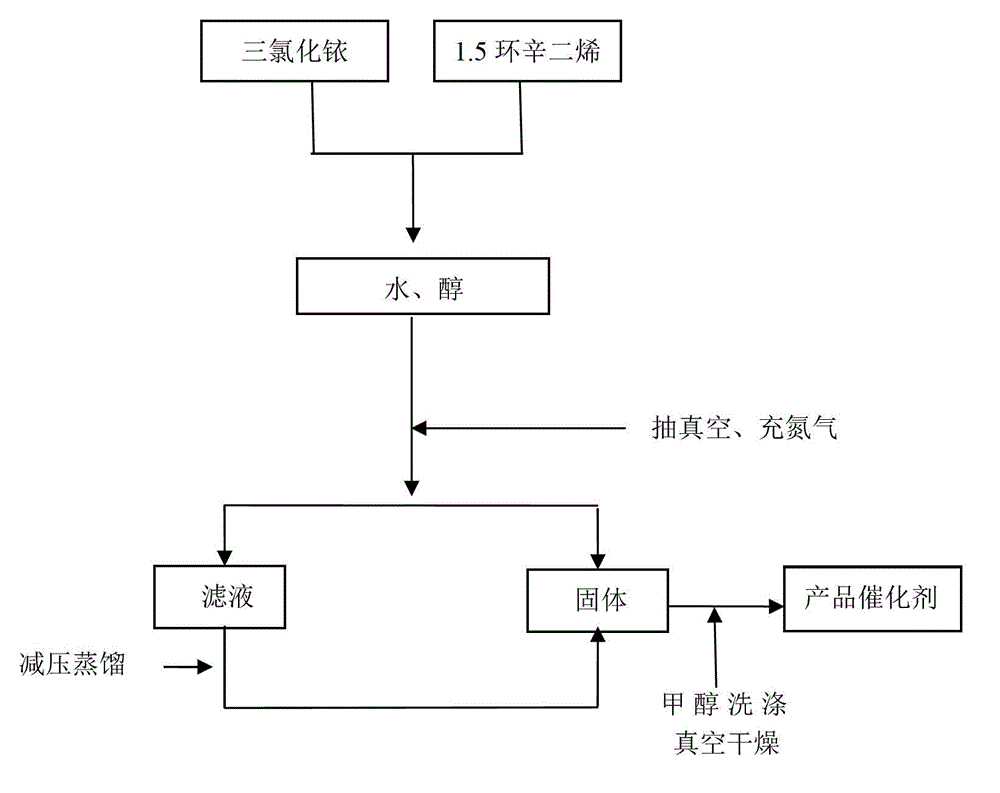
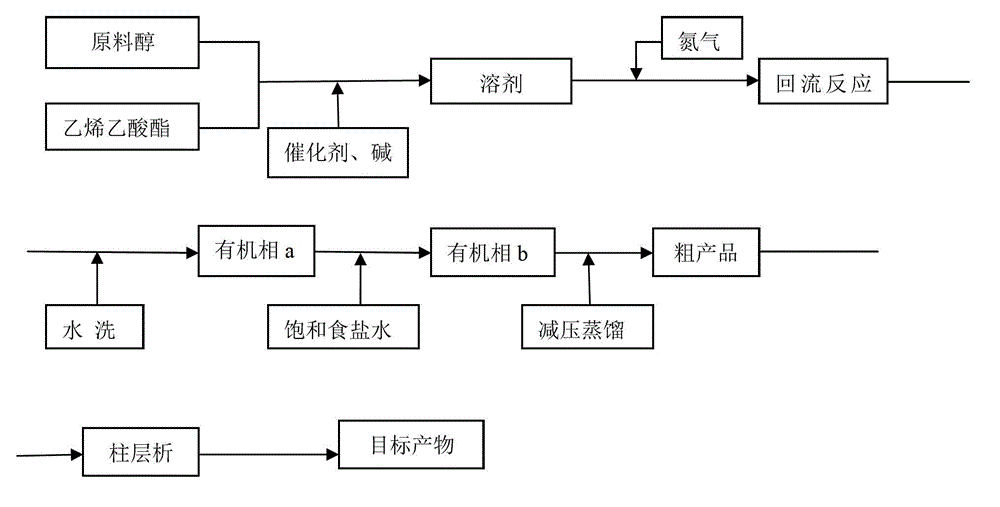
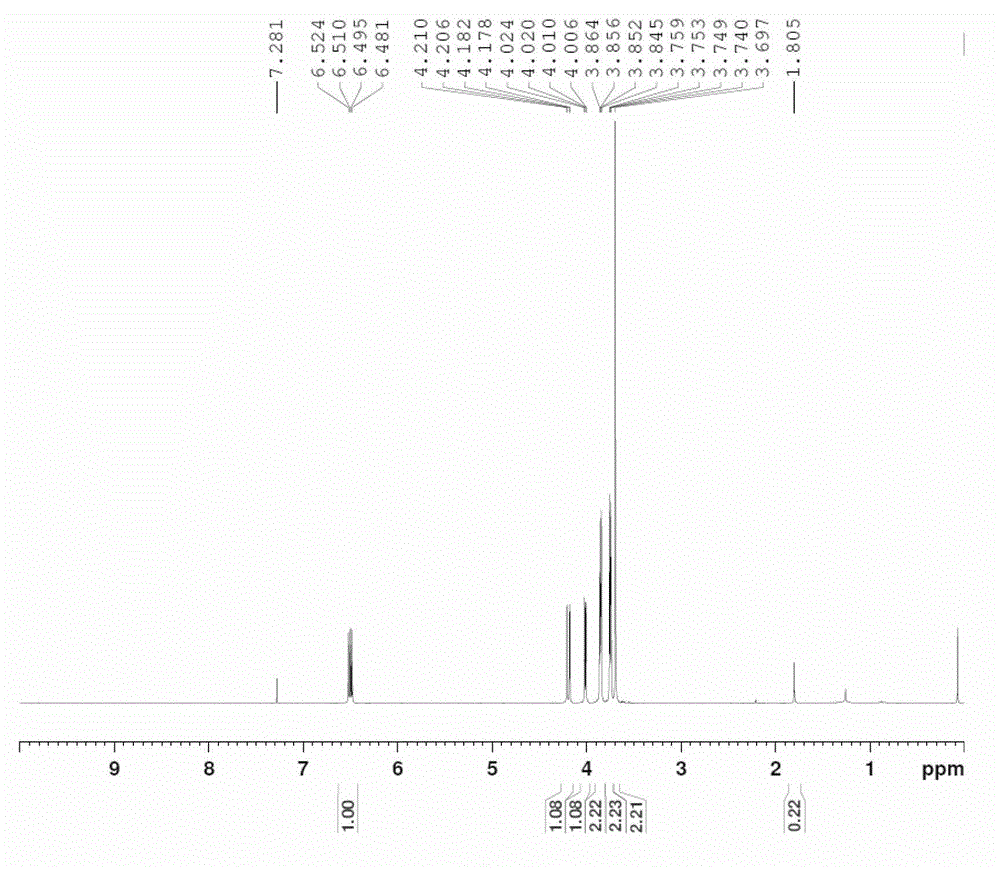
The invention discloses a preparation method of a vinyl ether compound. The vinyl ether compound is benzyloxy vinyl ether or triethyleneglycol divinyl ether. The preparation method is characterized by comprising the following steps: 1, preparing a catalyst [Ir(COD)Cl]2, wherein the catalyst [Ir(COD)Cl]2 is prepared by dissolving iridous chloride and 1,5-cyclooctadiene in water and alcohol and performing reflux reaction under waterless and oxygen-free conditions; 2, dissolving the catalyst [Ir(COD)Cl]2 and raw materials such as alcohol, vinyl acetate and sodium carbonate, and performing reflux reaction under a waterless and oxygen-free atmosphere; and 3, after the reaction is finished, washing with water, performing reduced pressure distillation, and performing column chromatography to obtain the product. The preparation method effectively solves the problems of high catalyst cost, high reaction temperature, complicated operation, high solvent toxicity and the like in the prior art, thereby providing a green preparation method of a vinyl ether compound, which has the advantages of high yield and simple operation steps.
Owner:NANJING UNIV OF SCI & TECH
Iridium complex electroluminescent material, preparation method and application thereof
InactiveCN111892631AImprove luminous efficiencyLong luminous lifeIndium organic compoundsSolid-state devicesEthylene glycol monoethyl etherOrganic electroluminescence
The invention discloses an iridium complex electroluminescent material. The specific structural general formula of the iridium complex electroluminescent material is shown as the specification. The preparation method comprises the following steps of: 1, adding a raw material A and iridium trichloride into a mixed solvent of ethylene glycol monoethyl ether / water, and carrying out full reaction to prepare a bridging ligand intermediate B; and 2, adding potassium carbonate and ethylene glycol monoethyl ether into the intermediate B and an intermediate C, and carrying out full reaction to obtain the product. The invention also discloses an organic electroluminescent device containing the iridium complex electroluminescent material, the organic electroluminescent device comprises a first electrode, a second electrode and one or more organic matter layers arranged between the first electrode and the second electrode, and the organic matter layers contain the iridium complex electroluminescent material. According to the iridium complex electroluminescent material, the specific heterocyclic ligand is selected for combination, the wavelength of the compound is adjusted, and after the obtained iridium complex electroluminescent material is used for an organic electroluminescent device, the luminous efficiency of the device is remarkably improved, and the service life of the device is remarkably prolonged.
Owner:奥来德(上海)光电材料科技有限公司
Iridium metal complex luminescent material and preparation and application thereof
InactiveCN111825725ASimple processHigh purityIndium organic compoundsSolid-state devicesOrganic electroluminescenceOrganic chemistry
The invention discloses an iridium metal complex luminescent material and preparation and application thereof, and belongs to the technical field of organic luminescent materials. The structural general formula of the iridium metal complex luminescent material provided by the invention is as shown in a formula I, and the target product as shown in the formula I is obtained by reacting a compound as shown in a formula II with iridium trichloride to obtain a bridging ligand compound as shown in a general formula IV and then reacting the compound as shown in the formula IV with a compound as shown in a general formula III. When the iridium metal complex provided by the invention is applied to the organic light-emitting device, the light-emitting efficiency of the light-emitting device can beimproved, and meanwhile, the service life of the light-emitting device is prolonged.
Owner:奥来德(上海)光电材料科技有限公司
Method for preparing iridous chloride hydrate
ActiveCN102408135BThe dissolution process is simpleHigh purityRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsChlorideAqueous solution
The invention relates to a method for preparing an iridous chloride hydrate (IrCl3.3H2O). The method comprises the following steps of: adding a hydrochloric acid solution and an iridium powder raw material into a U-shaped electrolytic cell, loading alternating current onto the two ends of an electrode, and directly dissolving the iridium powder into hydrochloric acid to obtain a chloroiridic acid aqueous solution; filtering the chloroiridic acid aqueous solution, and distilling to obtain a chloroiridic acid concentrated solution; and crystallizing the chloroiridic acid concentrated solution in a crystallization furnace to obtain an iridous chloride hydrate. In the preparation method of the iridous chloride hydrate, the iridous powder raw material is directly dissolved into the hydrochloric acid without adding any other reagent, so that the production cost is lowered, and a product is crystalized into the iridous chloride hydrate which is easy to carry, has stable iridium concentration, and is convenient to weigh and use; and when a high-purify iridous powder raw material is adopted, the impurity interference can be prevented, and a high-purity iridous chloride hydrate is prepared.
Owner:CHINA PETROLEUM & CHEM CORP +1
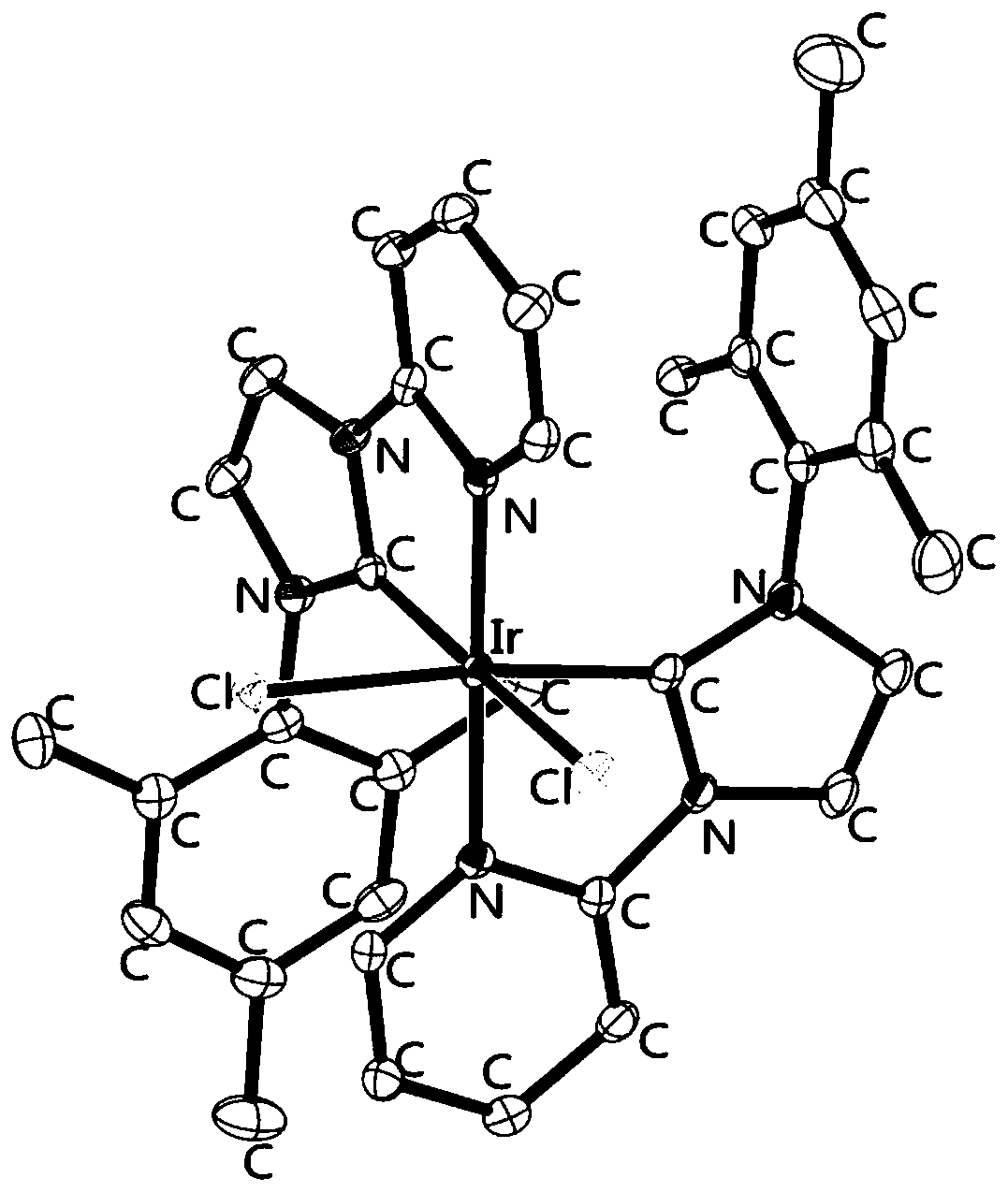
Metal iridium-carbene complex with photocatalytic performance as well as preparation method and application thereof
InactiveCN111548372AImprove photocatalytic efficiencyImprove catalytic performanceIndium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPotassium hexafluorophosphateOrganic base
The invention relates to a metal iridium-carbene complex with photocatalytic performance as well as a preparation method and application thereof, the molecular formula of the metal iridium-carbene complex is C34H36Cl2IrN6PF6, and the metal iridium-carbene complex is a mononuclear-hexadentate coordination compound of a monoclinic system and belongs to a P21 / c space group; the preparation method comprises the following steps: firstly, preparing carbene precursor pyridine-imidazole bromide from N-(2, 4, 6-trimethylphenyl) imidazole and 2-bromopyridine, then reacting with silver oxide to prepare asilver-carbene intermediate complex, and finally reacting with iridium trichloride and potassium hexafluorophosphate to prepare the metal iridium-carbene complex. The complex is applied to an addition reaction of a terminal alkyne compound and an isatin derivative, a visible light source is additionally arranged, and the addition reaction can be well carried out at room temperature by weak organic base; the metal iridium-carbene complex disclosed by the invention is relatively high in efficiency when being used as a photocatalyst for the addition reaction, and the yield of an addition productis 82 wt% or above.
Owner:CHANGZHOU INST OF TECH
Method for preparing trisacetylacetonate iridium through solid phase synthesis
InactiveCN106631743AReduce pollutionThe reaction steps are simplePreparation of aldehyde/ketone chelatesSolventCoordination complex
The invention discloses a method for preparing trisacetylacetonate iridium through solid phase synthesis. The method comprises: grinding acetylacetone and sodium carbonate according to a certain ratio, adding a certain amount of iridium trichloride hydrate and a reducing agent for grinding to obtain solid powder, and performing heating, secondary grinding, cooling, washing and drying. The yield can reach 55-60%. The method is mild in condition, friendly to the environment, easy to operate, and high in yield. The adverse effect of a solvent on a functional complex in a reaction operation process is prevented, and the method is suitable for massive production of trisacetylacetonate iridium.
Owner:KUNMING UNIV OF SCI & TECH
Organic metal iridium complex luminescent material and synthetic method of material
InactiveCN103172677AImprove solubilityImprove transfer rateGroup 8/9/10/18 element organic compoundsLuminescent compositionsOrganic layerNitrogen gas
The invention discloses an organic metal iridium complex luminescent material and a synthetic method of the material. The synthetic method disclosed by the invention comprises the following steps of: mixing an organic complex with iridium chloride hydrate in nitrogen atmosphere, subsequently adding a mixed solvent of tetrahydrofuran and water, heating in the nitrogen atmosphere and cooling to the room temperature; separating the reaction mixture liquid to acquire a tetrahydrofuran organic layer, and drying to remove the tetrahydrofuran solvent so as to acquire a metal iridium dimer complex; completely dissolving the metal iridium dimer complex and acetyl acetone thallium in dichloromethane and stirring at the room temperature in the nitrogen atmosphere; then purifying the metal iridium dimer complex on a chromatography silica gel column or a self-manufactured thin layer silica gel chromatography plate to acquire the final product which is the organic metal iridium complex luminescent material. According to the invention, the mixed solvent of the tetrahydrofuran and the water is taken as a reaction solvent for synthesizing the organic metal iridium complex, so that the synthetic yield of the organic metal iridium complex can be significantly improved; and the reaction after-treatment is simplified, so that the after-treatment time is shortened and the energy resources are saved.
Owner:XI AN JIAOTONG UNIV
High-selectivity reduction catalyst for nitro-aromatic hydrocarbon as well as preparation method and application of high-selectivity reduction catalyst
PendingCN113663670AConfinement stabilization of self-assembled structuresImprove stabilityOrganic compound preparationAmino compound preparationPtru catalystNitrobenzene
The invention belongs to the technical field of nano materials and preparation thereof, and particularly discloses a high-selectivity reduction catalyst for nitro-aromatic hydrocarbon as well as a preparation method and application of the high-selectivity reduction catalyst. Iridium trichloride is used as a metal precursor, isopropanol / ethanol is used as a solvent and a reducing agent, a hydroxyl-terminated hyperbranched polymer (H102) is used as a carrier, supramolecular self-assembly of H102 in isopropanol / ethanol is utilized, a N2 introduction state is kept, an oil bath reaction is performed for 1 h at the temperature of 100 DEG C, and an Ir@H102 compound is prepared; and the Ir@H102 is calcined in an inert atmosphere at the temperature of 350-400 DEG C (T) for 2 hours to prepare the novel composite nano material Ir@H102-T supported by a hyperbranched carbon skeleton. The Ir@H102-350 is applied to catalytic hydrogenation of nitro-aromatic hydrocarbon, nitrobenzene and derivatives thereof can be subjected to rapid and high-selectivity hydro-conversion into corresponding arylamine at room temperature, hydrogenation reaction is performed for 30 min at room temperature (25 DEG C) and under H2 pressure of 1.5 MPa, 100% conversion of nitro-aromatic hydrocarbon and 99.9% selectivity of corresponding arylamine can be achieved, and the catalyst is extremely good in stability and reusability and wide in industrial application prospect.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Organic iridium metal complex, preparation method and application thereof
PendingCN111333684AEasy to purifyImprove luminous efficiencyIndium organic compoundsSolid-state devicesOrganic electroluminescenceLuminescent material
The invention discloses an organic iridium metal complex, a preparation method and application thereof, and belongs to the technical field of organic light-emitting materials. The structural general formula of the organic iridium metal complex provided by the invention is shown as chemical formula 1, and the target product shown as the chemical formula 1 is obtained by reacting a compound shown asgeneral formula A with iridium trichloride to obtain a compound shown as a general formula B of a bridging ligand and then reacting the compound shown as the general formula B with a compound shown as general formula C. When the organic iridium metal complex provided by the invention is applied to organic light-emitting devices, the light-emitting efficiency and the light-emitting brightness of the light-emitting devices can be improved, thus facilitating the use.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
Preparation and application of cyclometalated iridium complex ratio type carbon monoxide fluorescent probe
InactiveCN113620997AGood choiceGood reproducibilityIndium organic compoundsFluorescence/phosphorescenceFluorescence spectraPhenanthroline
The invention belongs to the technical field of organic metal complex molecular fluorescent probes, and particularly relates to preparation and application of a cyclometalated iridium complex ratio type carbon monoxide fluorescent probe. The preparation method comprises the following steps: firstly, taking iridium trichloride and 2-aminopyridine as raw materials, and glycol ether and water as solvents to synthesize a cyclometalated iridium complex ; taking 4-hydroxy-3-nitrobenzaldehyde, 1, 10-phenanthroline-5, 6-diketone and ammonium acetate as raw materials and glacial acetic acid as a solvent, and reacting to obtain a phenanthroline-cinnamyl aldehyde ligand; and taking a phenanthroline-cinnamyl aldehyde ligand as a raw material, taking a metal iridium complex and the phenanthroline-cinnamyl aldehyde ligand as reactants, taking dichloromethane and methanol as reaction solvents, reacting to obtain an o-hydroxynitrobenzene-phenanthroline-iridium complex product, and separating and purifying to obtain the o-hydroxynitrobenzene-phenanthroline-iridium complex. The fluorescence spectrum of the o-hydroxy iridium complex disclosed by the invention generates red shift in a carbon monoxide system, the fluorescence intensity is gradually increased along with the concentration, and the fluorescence does not change along with the polarity of a solvent, amino acid and in-vivo common ions.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Nitrogen-containing ligand iridium complex as well as preparation method and application thereof
PendingCN113637033AExcellent optical propertiesGood biocompatibilityIndium organic compoundsFluorescence/phosphorescenceDimerFluoProbes
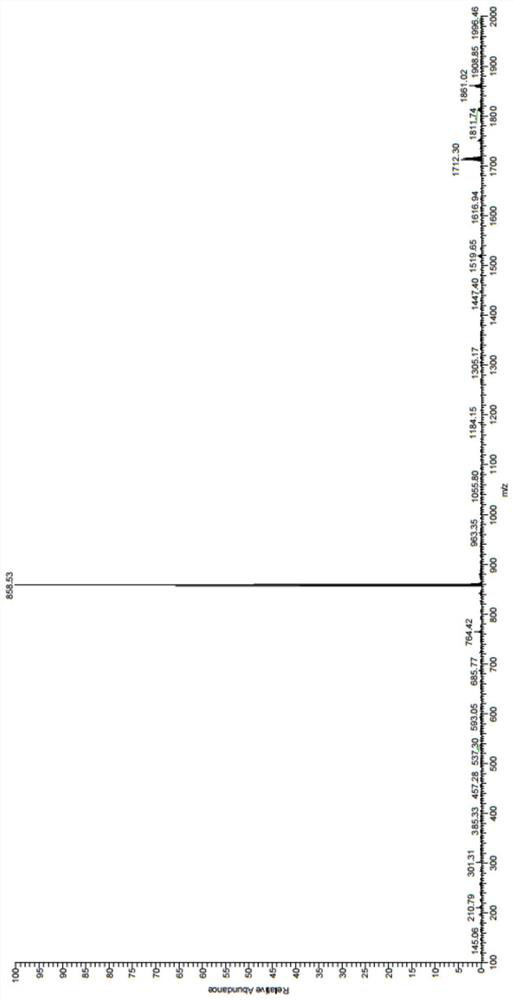
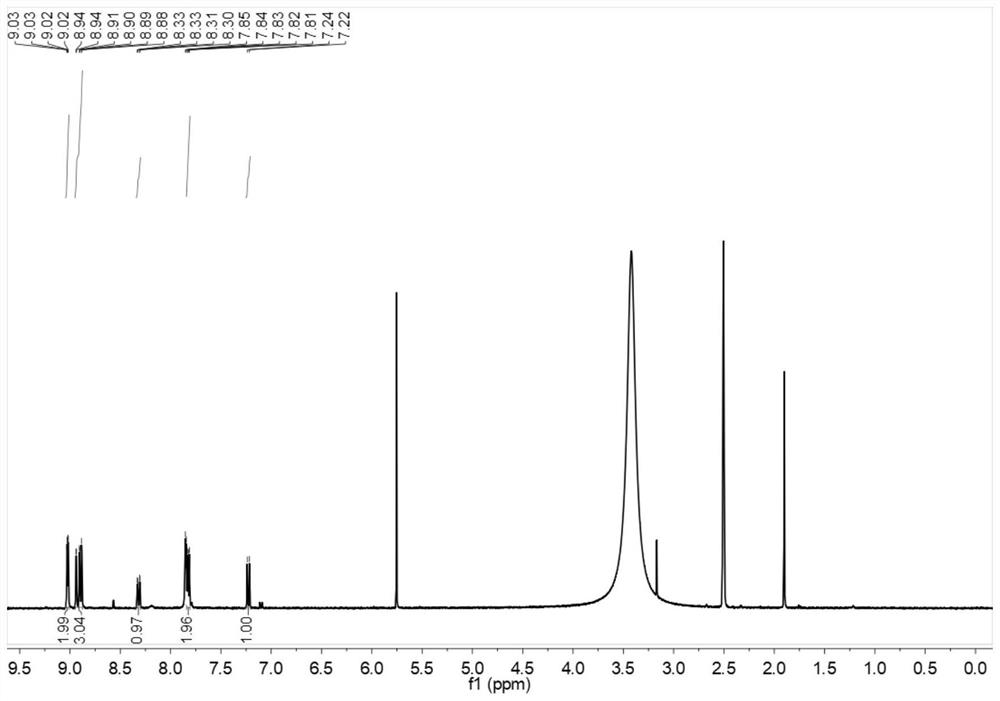
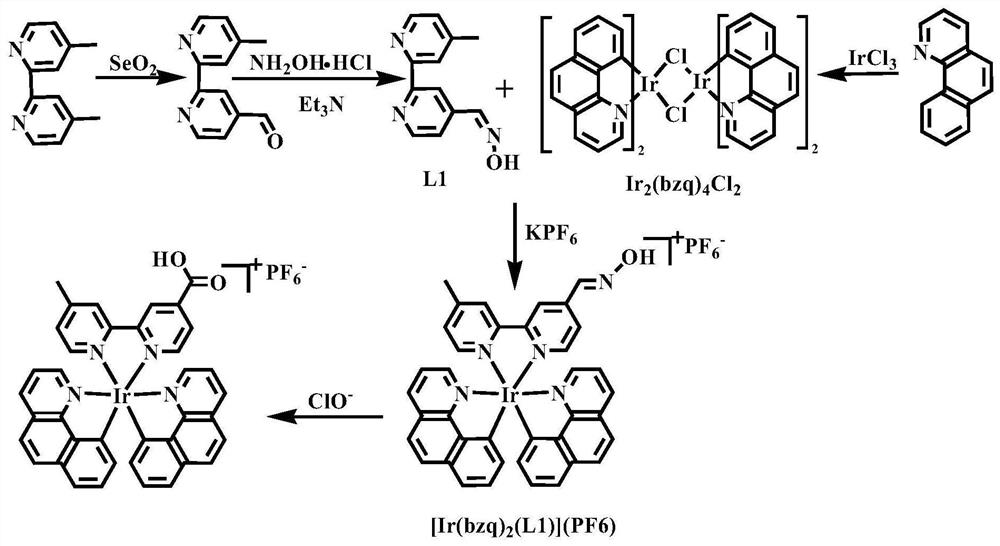
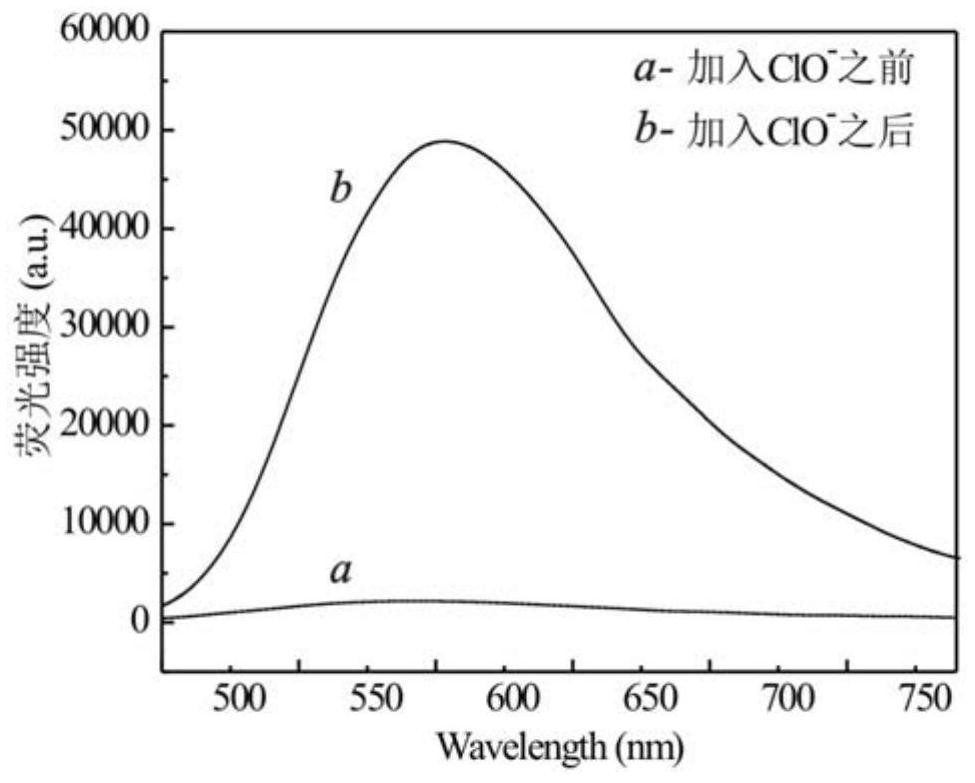
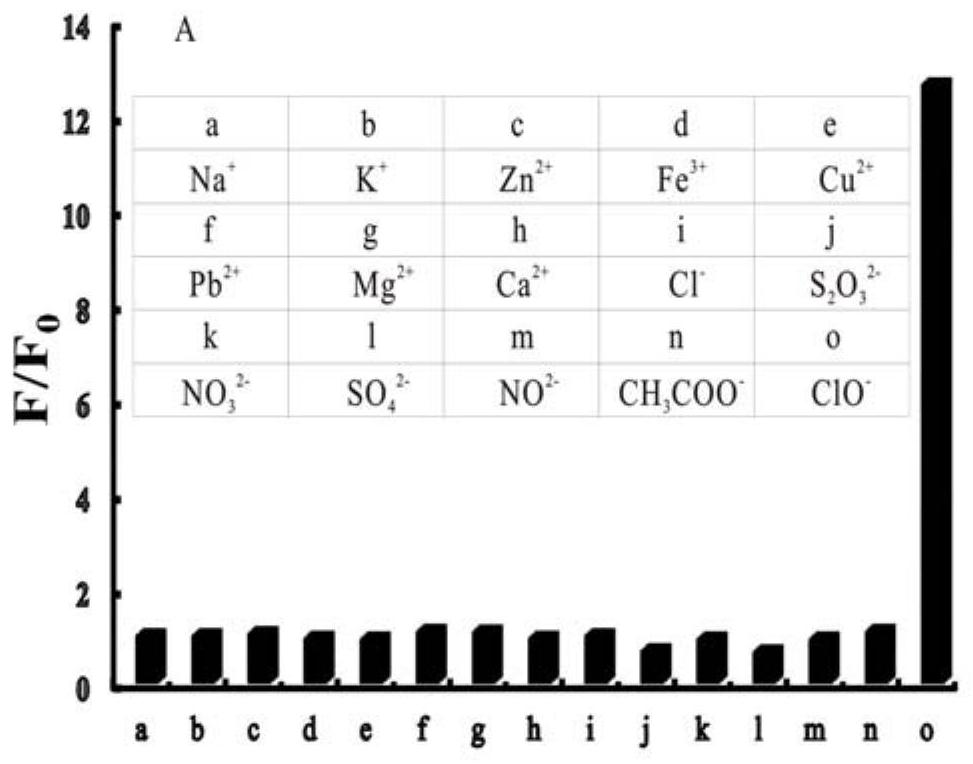
The invention provides a nitrogen-containing ligand iridium complex as well as a preparation method and application thereof, and belongs to the technical field of synthesis and application of inorganic materials. According to the iridium complex, firstly, a benzoquinoline iridium dimeric intermediate is synthesized from iridium trichloride and benzoquinoline, 4, 4'-dimethyl-2, 2'-bipyridyl is oxidized into bipyridine formaldehyde by using selenium dioxide at the same time, then hydroxylamine hydrochloride is added to reduce the bipyridine formaldehyde into bipyridine formaldehyde oxime, bipyridine formaldehyde oxime is used as a third ligand and reacts with the benzoquinoline iridium dimer to obtain a nitrogen-containing ligand iridium complex with a novel structure, and the nitrogen-containing ligand iridium complex can be used as a fluorescent probe for rapid identification and even trace detection of hypochlorite ions. The complex is simple in synthesis process, mild in reaction condition, small in reagent dosage, low in manufacturing cost, small in environmental pollution and good in optical property and biocompatibility. A hypochlorite ion fluorescent probe constructed by using the complex is high in sensitivity, good in selectivity, good in anti-interference performance, wide in concentration detection range and low in detection limit.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Method for synthesizing iridium (III) triacetylacetonate
The invention relates to a method for synthesizing iridium (III) acetylacetonate which comprises, dissolving iridium (III) chloride trihydrated into thermal distillation water, charing acetylacetone when agitating and letting in hydrogen gas, backflow, dropping saturated sodium bicarbonate solution, backflow, stopping hydrogen gas, filtering to obtain the end product.
Owner:KUNMING INST OF PRECIOUS METALS
A kind of synthetic method of iridium acetate
ActiveCN108409554BImprove conversion rateNo adverse side effectsOrganic compound preparationCarboxylic acid salt preparationPhysical chemistryEthylic acid
The invention discloses a synthesis method of iridium acetate. The method comprises: 1. dissolving iridium trichloride in water to form iridium trichloride solution and heating; 2. dissolving silver acetate in acetic acid solution to form silver acetate solution, Then silver acetate solution is added dropwise in the heated iridium chloride solution, reflux reaction under constant temperature, obtains the slurry that contains precipitation; 3, filter slurry to obtain filtrate and filter residue, wash filter residue with water and wash liquid into the filtrate to obtain the mother liquor, and then the mother liquor is successively concentrated and dried to obtain iridium acetate. The invention does not need to melt the iridium powder with alkali, directly transfers the iridium-containing raw material into the liquid reaction system, and synthesizes iridium acetate through one-step reaction, which overcomes the problem of insufficient reaction caused by the limited solubility of iridium in the traditional process, and has no adverse side reactions. The purity of the method is high, the conversion rate of iridium acetate is greatly improved, and the method is simple, the process is easy to control, the pollution to the environment is small, and it is suitable for industrialization.
Owner:XIAN RAREALLOYS
Surface metal electroplating liquid of energy equipment
The invention discloses surface metal electroplating liquid of energy equipment. An additive and a carrier are included. Raw materials in a formula comprise, by weight percent, 0.5%-6% of lauryl sodium sulfate, 10%-35% of nickel aminosulfonate, 1%-10% of iridous chloride, 0%-3% of ammonium perrhenate, 0%-2.5% of alums, 5%-20% of a coloring agent, 10%-20% of sodium hydroxide and 30%-60% of a catalyst. A product prepared through the method can achieve the effects that toxicity and pollution are avoided, the electroplating technology is optimized, cost is low, and the surface metal electroplatingliquid of energy equipment can be applied to deposition of a rhenium-iridium alloy on the surface of a conductive base body complex in shape.
Owner:徐州金茂智慧能源科技有限公司
Fluorene and spiro-fluorene substituted phenylpyridine iridium complex and preparation method and application thereof
InactiveCN101974036BIncrease distanceImprove transmission performanceGroup 8/9/10/18 element organic compoundsSolid-state devicesSolventPhenyl group
The invention belongs to the field of organic electroluminescent materials and relates to a fluorene and spiro-fluorene substituted phenylpyridine iridium complex and a preparation method and application thereof. The preparation method comprises the following steps of: synthesizing various fluorene and spiro-fluorene substituted phenylpyridine iridium ligands; reacting the ligands with iridium(III) chloride hydrate (IrCl3.3H2O) to obtain a chlorine-bridged dimer intermediate; and reacting the intermediate with acetylacetone in a proper solvent to obtain the fluorene and spiro-fluorene substituted phenylpyridine iridium complex. The iridium complex can change types and positions of the substituent of phenyl in a bidentateligand, can adjust luminous wavelength in a certain range and can be widely applied in the field of organic electroluminescence.
Owner:EAST CHINA NORMAL UNIV
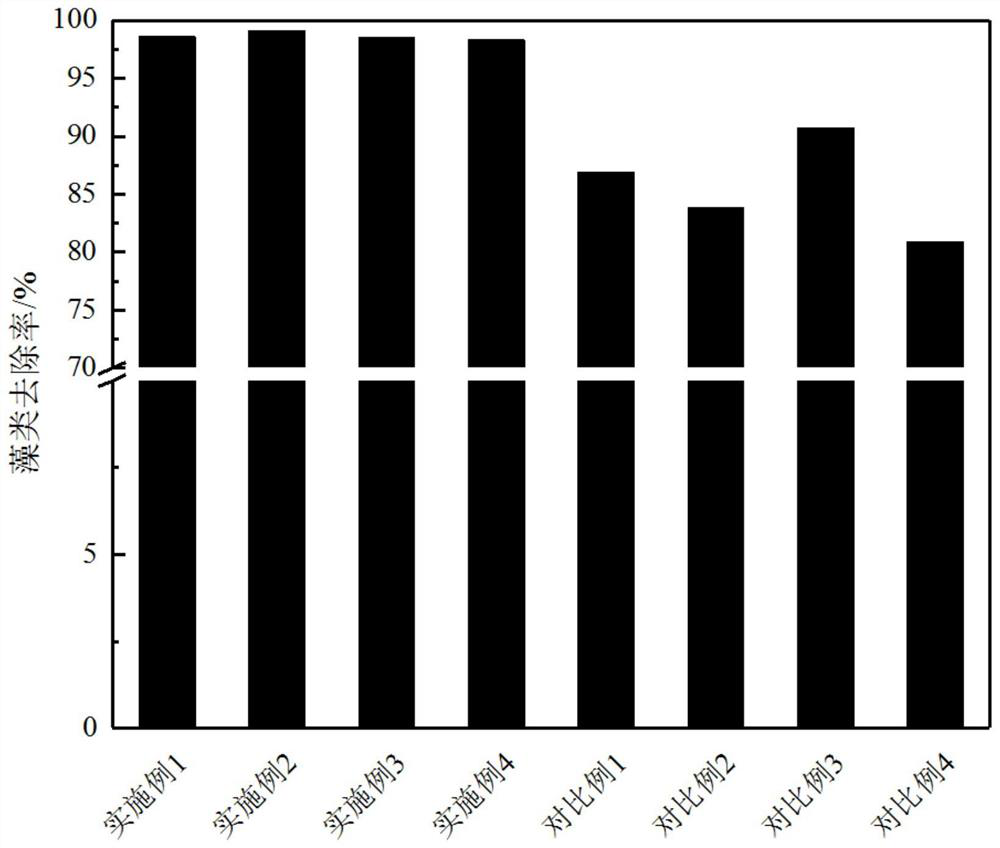
A kind of preparation method of environment-friendly algae-removing coagulant
ActiveCN113057176BGood effect on algae removalReduce pollutionBiocideAnimal repellantsAluminium chlorideWater chlorination
The invention discloses a preparation method of an environment-friendly algae-removing coagulant. The preparation method is as follows: (1) taking aluminum chloride hexahydrate, ferric chloride hexahydrate and cerium trichloride hexahydrate respectively after sieving Powder, mixed evenly, heated for one roasting, air cooling, mixed with iridium trichloride, second roasting, air cooling; (2) Add deionized water to the roasted powder to react until dark brown polymer is formed and air cool to room temperature Stop stirring to obtain the crude coagulant; (3) Configure an aqueous solution of sodium hydroxide, soak the crude coagulant in the aqueous solution of sodium hydroxide, stir rapidly, then add iron oxide powder and hydrogen peroxide sequentially therein, and the feeding is completed Then continue to stir, stand at room temperature for more than 20h, and freeze-dry to obtain the coagulant. The algae-removing coagulant prepared by the invention has good algae-removal effect, less water pollution, and is suitable for primary treatment of water body pollutants.
Owner:CHANGZHOU INST OF LIGHT IND TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


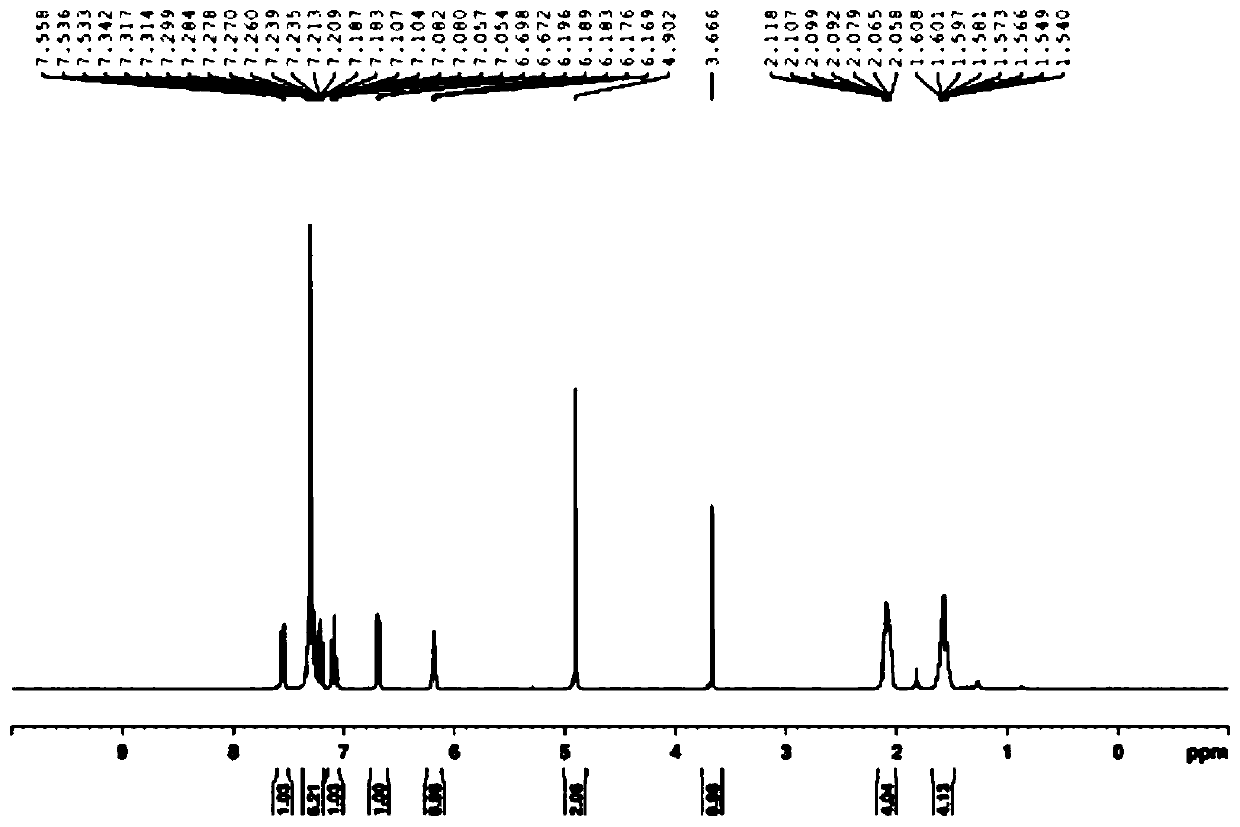
![4,7-diarylthieno[2, 3-d] pyridazine cyclometalated iridium complex and preparation method thereof 4,7-diarylthieno[2, 3-d] pyridazine cyclometalated iridium complex and preparation method thereof](https://images-eureka.patsnap.com/patent_img/d028355e-9ae2-4ddb-8283-a1e899df650e/HDA0002462683680000011.png)
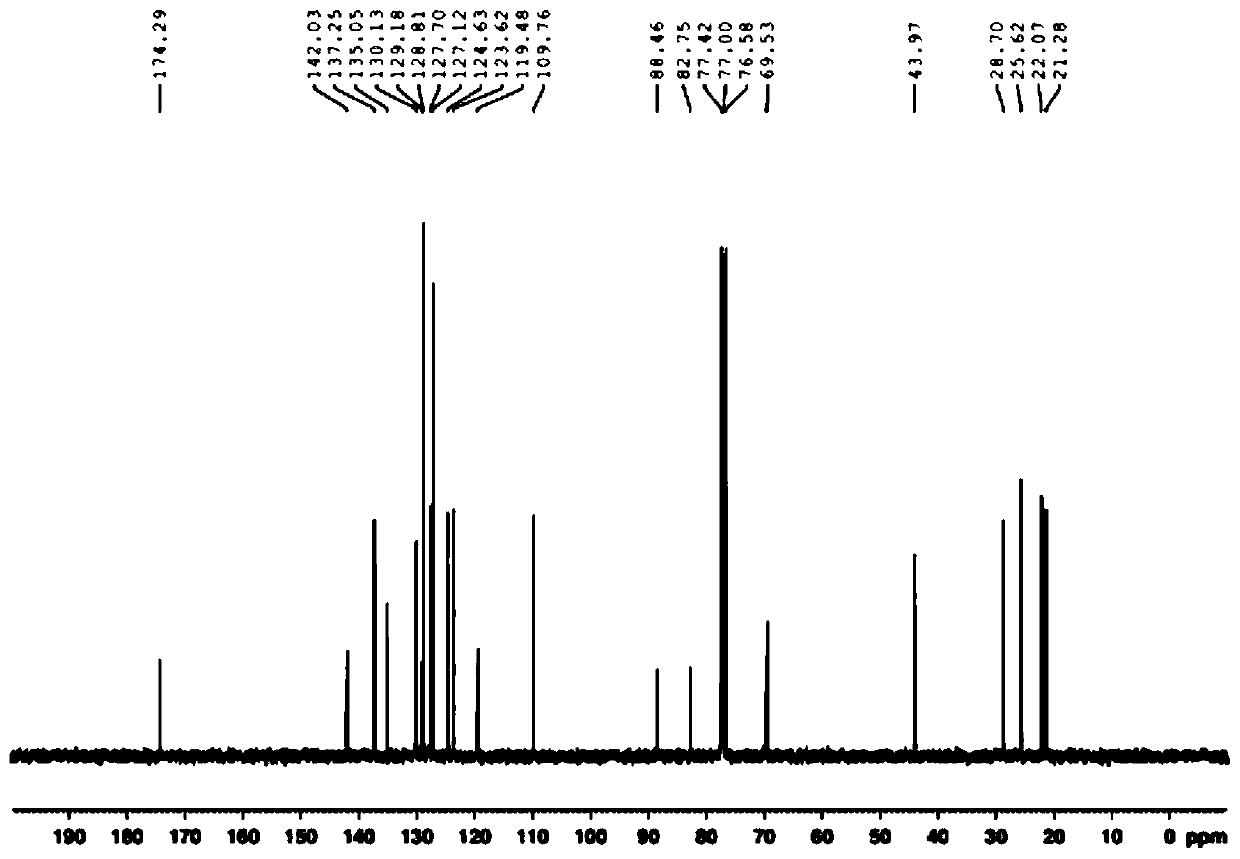
![4,7-diarylthieno[2, 3-d] pyridazine cyclometalated iridium complex and preparation method thereof 4,7-diarylthieno[2, 3-d] pyridazine cyclometalated iridium complex and preparation method thereof](https://images-eureka.patsnap.com/patent_img/d028355e-9ae2-4ddb-8283-a1e899df650e/HDA0002462683680000012.png)
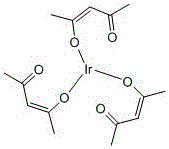
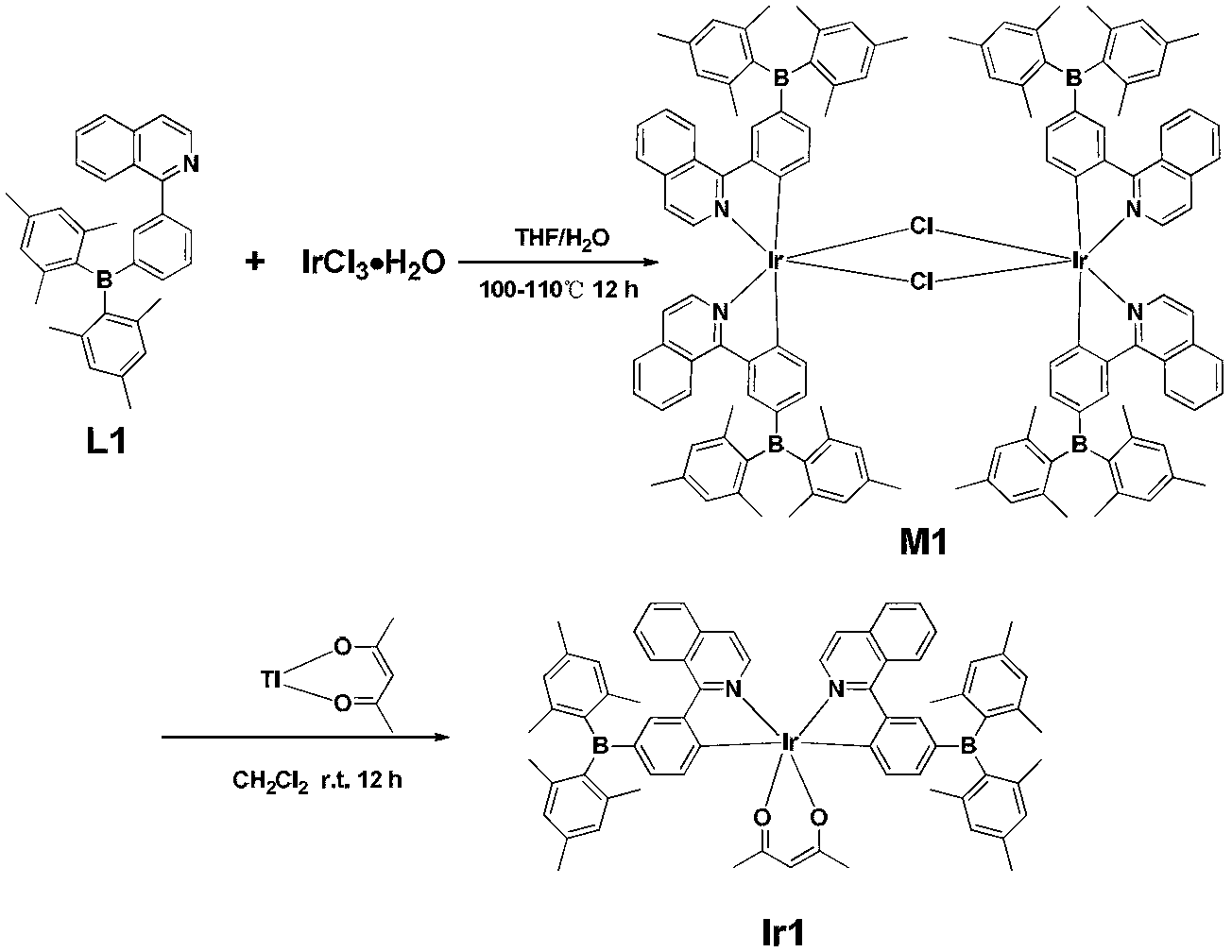
![4,7-diarylthieno[2, 3-d] pyridazine cyclometalated iridium complex and preparation method thereof 4,7-diarylthieno[2, 3-d] pyridazine cyclometalated iridium complex and preparation method thereof](https://images-eureka.patsnap.com/patent_img/d028355e-9ae2-4ddb-8283-a1e899df650e/HDA0002462683680000013.png)