Method for preparing iridous chloride hydrate
A technology of iridium trichloride and hydrate, which is applied in the field of preparation of iridium trichloride hydrate IrCl 3H2O, can solve the problems of low purity of iridium trichloride hydrate, increase preparation steps, increase iridium loss, etc. The effect of high iridium yield, high product purity and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The preparation method of iridium chloride hydrate of the present invention comprises the following steps:
[0050] Step 1, electrolysis: add hydrochloric acid solution 4 and iridium powder raw material 5 in U-shaped electrolytic cell 1;
[0051] The U-shaped electrolytic cell 1 is made of an acid-resistant material, and an electrode 2 of a non-metallic conductive material is arranged in the U-shaped electrolytic cell;
[0052] Control the temperature of the hydrochloric acid in the electrolytic cell at 100-115°C, apply an alternating current with a voltage of 5-80 volts and a current of 2-50 amperes at both ends of the electrodes, and perform constant voltage electrolysis until the iridium powder dissolves to form an aqueous solution of chloroiridic acid ;
[0053] The purity of said hydrochloric acid is superior grade pure, and the concentration of hydrochloric acid is 8~12mol / L; The weight ratio of hydrochloric acid and iridium powder is 3:1~100:1; High concentrati...
Embodiment 1
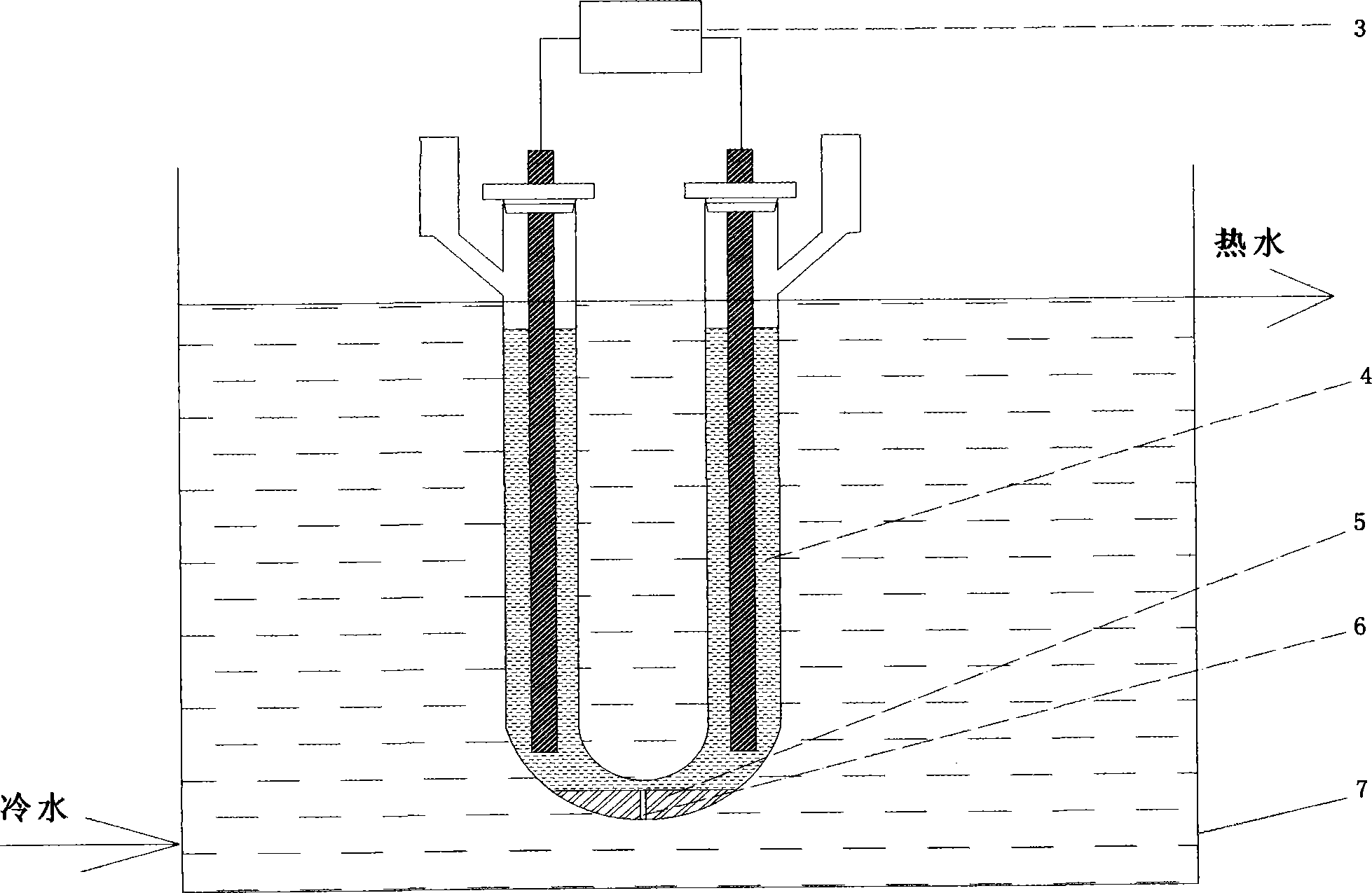
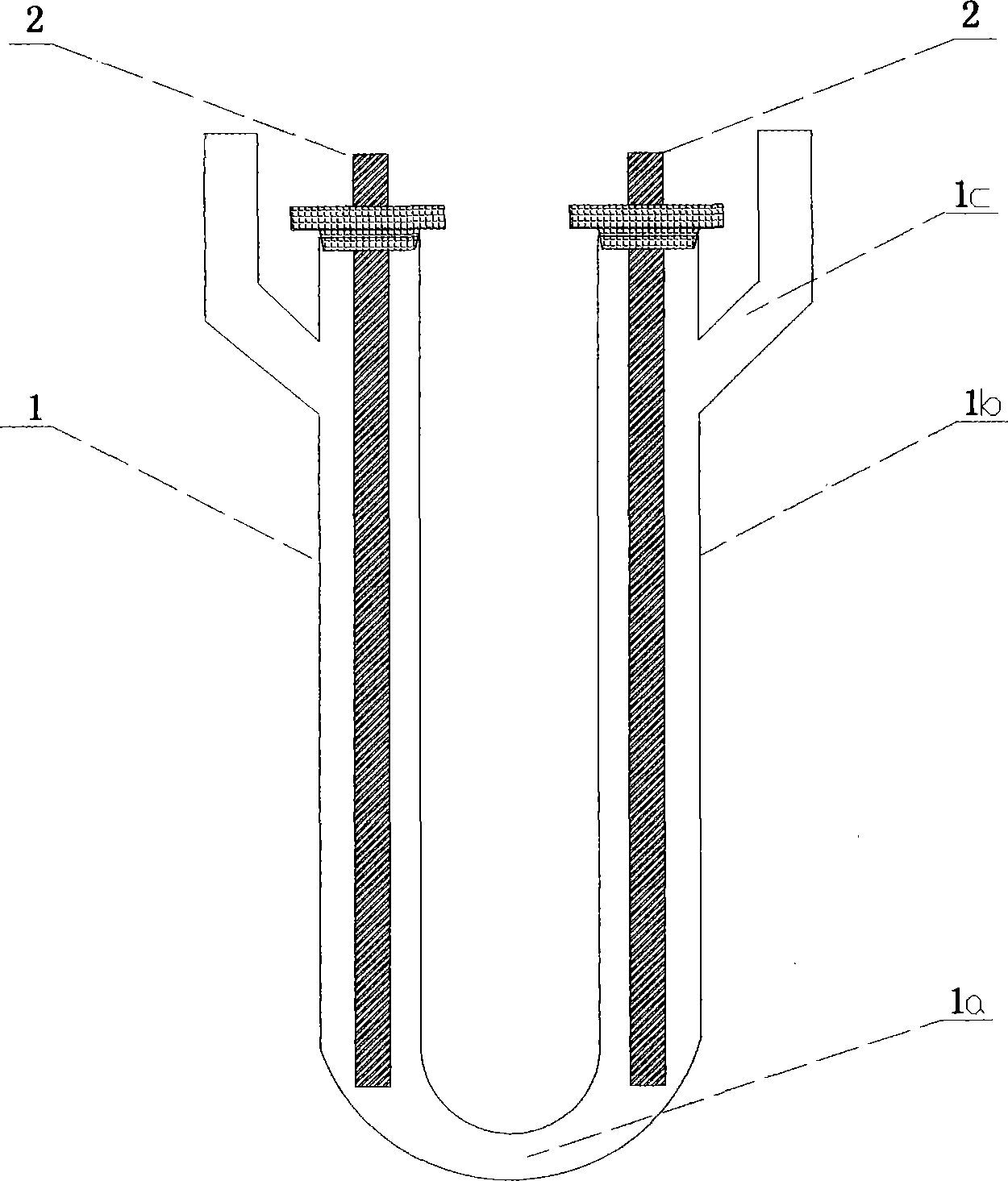
[0086] figure 1 is a schematic diagram of a U-shaped electrolytic cell. The diameter of U-shaped quartz electrolytic cell 1 is 50mm for the standpipe 1b, and the distance between the centers of the two standpipes is 180mm; the diameter of the bottom bend 1a is 18mm, and the diameter of the standpipe: the diameter of the bend=2.78; The length is 25mm; the volume of the electrolytic cell is 800ml; the electrode 2 is a pure graphite electrode with a length of Φ12mm and a length of 250mm. A cold water condenser is installed on both sides of the U-shaped quartz electrolytic cell 1 through the condenser interface 1c.
[0087] Iridium powder dissolving device see figure 2 , wherein: the two ends of the graphite electrode are connected to the AC arc generation controller 3, the iridium powder 5 is placed in the bottom elbow 1a of the U-shaped electrolytic cell, and the hydrochloric acid solution 4 is added to the electrolytic cell 1 and placed in the circulating cooling water tank ...
Embodiment 2、3
[0090] Change the voltage of the electrolytic alternating current to 45 volts and 55 volts respectively, and the magnitude of the current varies from 10 to 40 amps. Other conditions are as shown in Example 1. The dissolution rates of iridium powder were 89.0% and 85.2% respectively. The solution after the electrolysis of Examples 2 and 3 was filtered, the filtrates were combined, and through simple distillation, excess hydrochloric acid was distilled to obtain 2.1M / L chloroiridic acid H 3 IrCl 6 solution. H 3 IrCl 6 The concentrated solution was placed in a crystallization furnace, and crystallized at 115 °C for 10 h to obtain 95.9 g of iridium trichloride hydrate (IrCl 3 ·3H 2 O). The weight percent content of Ir analyzed by gravimetric method is 54.48%. According to the analysis value of iridium content, the yield of iridium is greater than 99.9%. The total weight percentage content of ten kinds of impurity metals such as K, Na, Ca, Si, Rh, Au, Ag, Mg, Pt, Cu etc. i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com