Preparation and application of cyclometalated iridium complex ratio type carbon monoxide fluorescent probe
An iridium complex, carbon monoxide technology, applied in fluorescence/phosphorescence, indium organic compounds, platinum group organic compounds, etc., can solve the problems of inability to track biological samples for a long time, poor spectral stability, etc., and achieve good selectivity and reproducibility , high sensitivity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
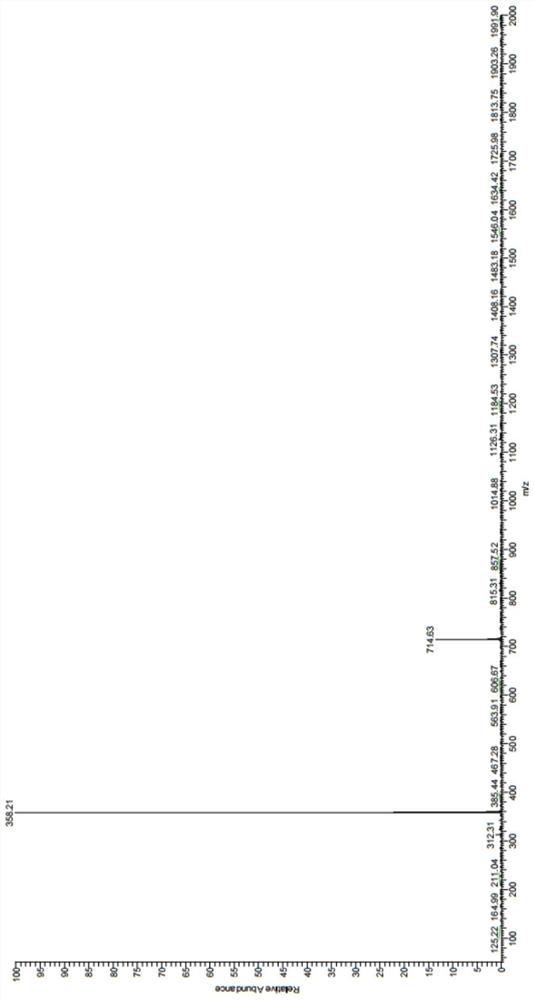
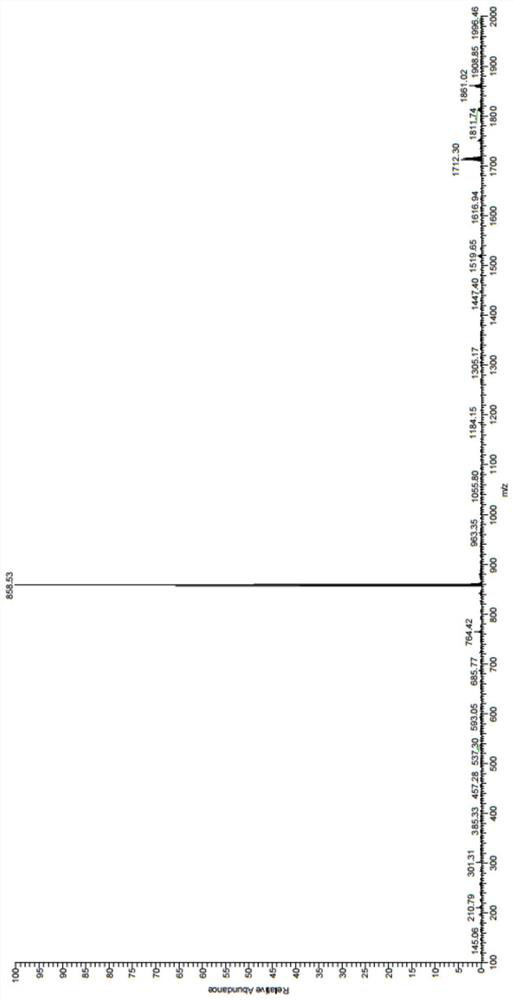
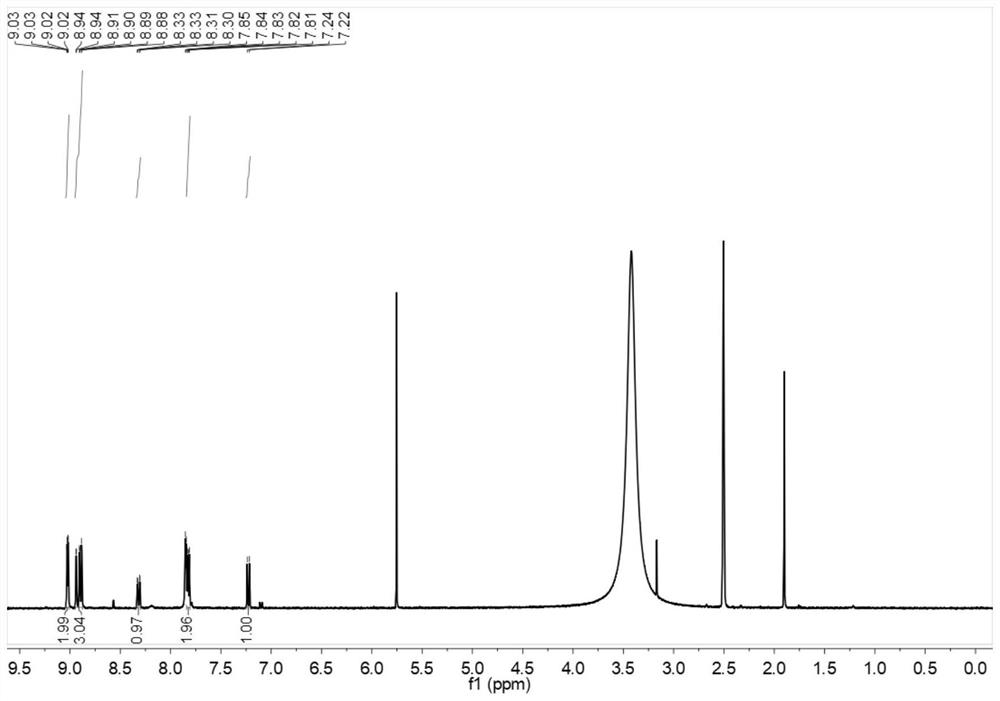
[0044] Synthesis of fluorescent probes for o-hydroxynitrobenzene-o-phenanthroline-iridium complexes:
[0045] Weigh 200 mg of iridium trichloride and 52 mg (48 μL) of 2-aminopyridine into a 100 ml round-bottomed flask, add 15 ml of ethylene glycol ether and 5 ml of water as a solvent, the reaction temperature is 135 ° C, and condense to reflux. The reaction time is 24h. After the reaction, cool to room temperature, ultrasonic and suction filter to obtain a yellow-green solid, which is finally washed with distilled water and dried to obtain a cyclometal iridium complex;
[0046] Weigh 150mg of 4-hydroxy-3-nitrobenzaldehyde, 158mg of 1,10-phenanthroline-5,6-dione, and 1160mg of ammonium acetate in a flask, add 15ml of glacial acetic acid as a solvent, and the reaction temperature is 118°C, condensing reflow. The reaction time is 5 hours. After the reaction, cool to room temperature, add a small amount of distilled water, slowly drop ammonia water into the flask, adjust the pH to...
Embodiment 2
[0050] Fluorescence intensities of o-hydroxynitrobenzene-o-phenanthroline-iridium complex fluorescent probes in different solvents:
[0051] Weigh 0.5 mg o-hydroxyiridium complex and dissolve it in 615 μL dimethyl sulfoxide to obtain 1 mmol / L o-hydroxyiridium complex mother solution. Take 4 μL o-hydroxyiridium complex mother solution (1 mmol / L) in a centrifuge tube, add dichloromethane, acetonitrile, dimethyl sulfoxide, dioxane, methanol, and dilute to 100 μL, and the liquid in the centrifuge tube Transfer to a 96-well black plate for analysis using a microplate reader (fixed excitation: 405nm, emission start: 450nm, stop: 700nm, step: 2nm). Among them, the solution in which the solvent is dichloromethane is extremely corrosive, and the 96-well plate is severely corroded, so the detection of dichloromethane is canceled. After detecting the fluorescence intensity, immediately transfer the liquid in the black plate to a transparent 96-well transparent plate to test the UV absor...
Embodiment 3
[0054] Fluorescence and UV absorption spectrometry of o-hydroxynitrobenzene-o-phenanthroline-iridium complex stimulated by carbon monoxide:
[0055] Weigh 0.5 mg of the o-hydroxyiridium complex and dissolve it in 615 μL of methanol to obtain a 1 mmol / L mother solution of the hydroxyiridium complex. Take 5 mg of ruthenium tricarbonyl dichloride dimer and dissolve it in 974 μL of dimethyl sulfoxide to obtain a 10 mmol / L mother solution of ruthenium tricarbonyl dichloride dimer solution.
[0056] 1. For different concentrations, take three 1.5ml centrifuge tubes, numbered 1, 2, and 3. Add 20 μL o-hydroxyiridium complex mother solution, 30 μL methanol, 50 μL dimethyl sulfoxide and 400 μL distilled water to number 1; add 20 μL hydroxyl iridium complex mother solution, 30 μL methanol, 2 μL tricarbonyl dichlororuthenium dimer to number 2 (10mmol / L), 48μL dimethyl sulfoxide and 400μL distilled water; add 20μL hydroxyiridium complex mother solution, 30μL methanol, 20μL tricarbonyl dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com