High-selectivity reduction catalyst for nitro-aromatic hydrocarbon as well as preparation method and application of high-selectivity reduction catalyst
A nitroaromatic, high-selectivity technology, applied in the preparation of amino compounds, preparation of amino hydroxyl compounds, chemical instruments and methods, etc., to achieve the effects of good stability, broad industrial application prospects and low carbonization temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kind of synthetic method of nitroaromatics highly selective reduction catalyst of the present embodiment, comprises the steps:
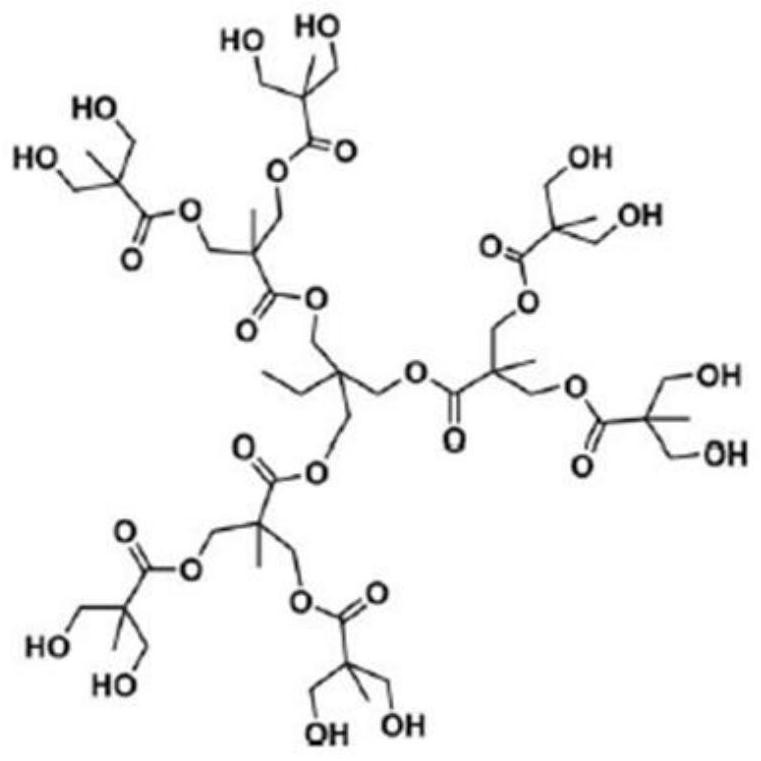
[0047] (1) Preparation of Ir@H102 complex: weigh 210mg NaHCO 3 and 1180mg H102 in a 50mL three-neck round bottom flask, add 15mL isopropanol, stir for 30min until the hyperbranched product is completely dissolved; then add 5mL 0.1mol L -1 IrCl 3 isopropanol solution, stirred for 30 minutes to make the solution evenly mixed; the mixed solution was ultrasonically treated for 10 minutes, and then passed into N 2 Remove the air; finally, put the reaction bottle into the oil bath and keep it open to N 2 state, react at a constant temperature of 100°C for 60 minutes; stop the reaction, and cool to room temperature. The isopropanol was removed by rotary evaporation at 45°C to obtain the Ir@H102 complex.
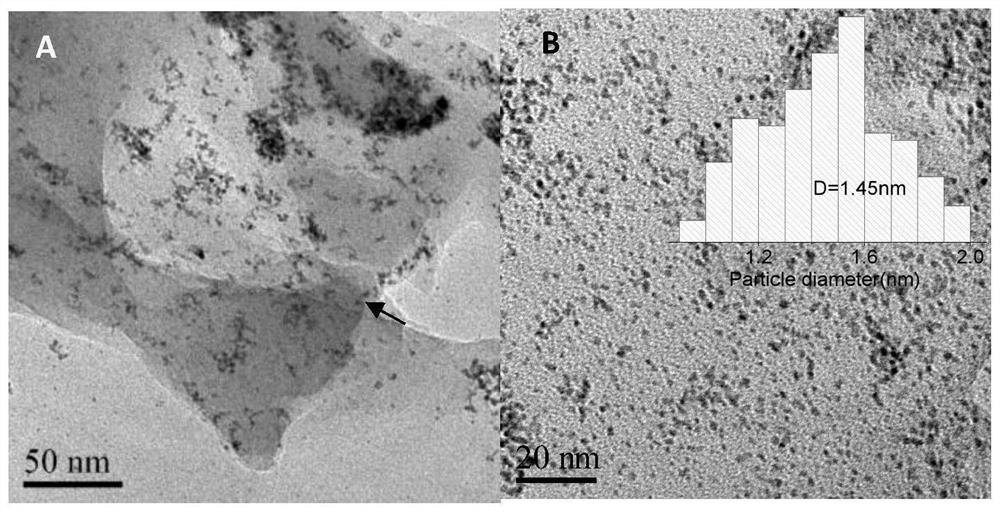
[0048] (2) Preparation of Ir@H102-350 catalyst: Transfer the Ir@H102 composite to a porcelain boat, place it in a constant temperature blast ...
Embodiment 2
[0056] A kind of synthetic method of nitroaromatics highly selective reduction catalyst of the present embodiment, comprises the steps:
[0057] (1) Preparation of Ir@H102 complex: weigh 210mg NaHCO 3 and 1180mg H102 in a 50mL three-neck round bottom flask, add 15mL isopropanol, stir for 30min until the hyperbranched product is completely dissolved; then add 5mL 0.1mol L -1 IrCl 3 isopropanol solution, stirred for 30 minutes to make the solution evenly mixed; the mixed solution was ultrasonically treated for 10 minutes, and then passed into N 2 Remove the air; finally, put the reaction bottle into the oil bath and keep it open to N 2 state, react at a constant temperature of 100°C for 60 minutes; stop the reaction, and cool to room temperature. The isopropanol was removed by rotary evaporation at 45°C to obtain the Ir@H102 complex.
[0058] (2) Preparation of Ir@H102-400 catalyst: Transfer the Ir@H102 composite to a porcelain boat, place it in a constant temperature blast ...
Embodiment 3
[0061] A kind of synthetic method of nitroaromatics highly selective reduction catalyst of the present embodiment, comprises the steps:
[0062] (1) Preparation of Ir@H102 complex: weigh 210mg NaHCO 3 and 1180mg H102 in a 50mL three-neck round bottom flask, add 15mL isopropanol, stir for 30min until the hyperbranched product is completely dissolved; then add 5mL 0.1mol L -1 IrCl 3 isopropanol solution, stirred for 30 minutes to make the solution evenly mixed; the mixed solution was ultrasonically treated for 10 minutes, and then passed into N 2 Remove the air; finally, put the reaction bottle into the oil bath and keep it open to N 2 state, react at a constant temperature of 100°C for 60 minutes; stop the reaction, and cool to room temperature. The isopropanol was removed by centrifugation at room temperature to obtain the Ir@H102 complex.
[0063] (2) Preparation of Ir@H102-350C catalyst: Transfer the Ir@H102 composite to a porcelain boat, place it in a constant temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com