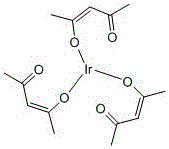
Method for preparing trisacetylacetonate iridium through solid phase synthesis
A solid-phase synthesis method and acetylacetone technology, applied in the field of catalysis, can solve the problems of low yield of iridium triacetylacetonate, etc., and achieve the effects of avoiding harsh conditions, reducing environmental pollution, and increasing reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Take 8.2mL of acetylacetone and 3.2g of sodium carbonate and grind them in a mortar, then add 3.5g of iridium trichloride hydrate and 2mmol of ascorbic acid and grind to form a paste until it is ground into a solid powder, heat it in a microwave at 800W for 10min, take out Then continue grinding, after cooling to room temperature, wash with distilled water, filter with suction, wash with methanol, and vacuum dry to obtain 2.72 g of iridium triacetylacetonate.
[0017] Productive rate=actual yield of iridium triacetylacetonate / theoretical yield of iridium triacetylacetonate=2.72g / 4.86g=55.9%.
[0018] Carry out result analysis to it, characteristic structure parameter: 1, 1 H-NMR (500MHz, DMSO): δ (ppm) = 5.43 (s, 2H, CH), 1.97 (s, 12H, CH 3 ); 2. MS: measured value m / z=490.87, theoretical value m / z=490.54; 3. Elemental analysis: measured value C 36.47%, H 4.27%, theoretical value C 36.76%, H 4.29%.
Embodiment 2
[0020] Take 12.4mL of acetylacetone and 6.8g of sodium bicarbonate and grind them in a mortar, then add 3.5g of iridium trichloride hydrate and 2mmol of ascorbic acid and grind to form a paste until it is ground into a solid powder, and heat it in a microwave oven at 950W for 9min. After taking it out, continue to grind, cool to room temperature, wash with distilled water, filter with suction, wash with methanol, and vacuum dry to obtain 2.89 g of iridium triacetylacetonate.
[0021] Productive rate=actual yield of iridium triacetylacetonate / theoretical yield of iridium triacetylacetonate=2.89g / 4.86g=59.5%.
[0022] Analyze the results, characteristic structure parameters: 1s, 1 H-NMR (500MHz, DMSO): δ (ppm) = 5.46 (s, 2H, CH), 1.95 (s, 12H, CH 3 ); 2. MS: measured value m / z=490.90, theoretical value m / z=490.54; 3. Elemental analysis: measured value C 36.68%, H 4.30%, theoretical value C 36.76%, H 4.29%.
Embodiment 3
[0024] Take 9.0mL of acetylacetone and 2.6g of sodium carbonate and grind them in a mortar, then add 3.5g of iridium trichloride hydrate and 1mmol of hydrazine hydrate and grind to form a paste until it is ground into a solid powder, and heat it in a microwave oven at 1100W for 9min. After taking it out, continue to grind, cool to room temperature, wash with distilled water, filter with suction, wash with methanol, and vacuum dry to obtain 2.86 g of iridium triacetylacetonate.
[0025] Productive rate=actual yield of iridium triacetylacetonate / theoretical yield of iridium triacetylacetonate=2.86g / 4.86g=58.8%.
[0026] Carry out result analysis to it, characteristic structure parameter: 1, 1 H-NMR (500MHz, DMSO): δ (ppm) = 5.45 (s, 2H, CH), 1.95 (s, 6H, CH 3 ); 2. MS: measured value m / z=490.75, theoretical value m / z=490.54; 3. Elemental analysis: measured value C 36.66%, H 4.30%, theoretical value C 36.76%, H 4.29%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com