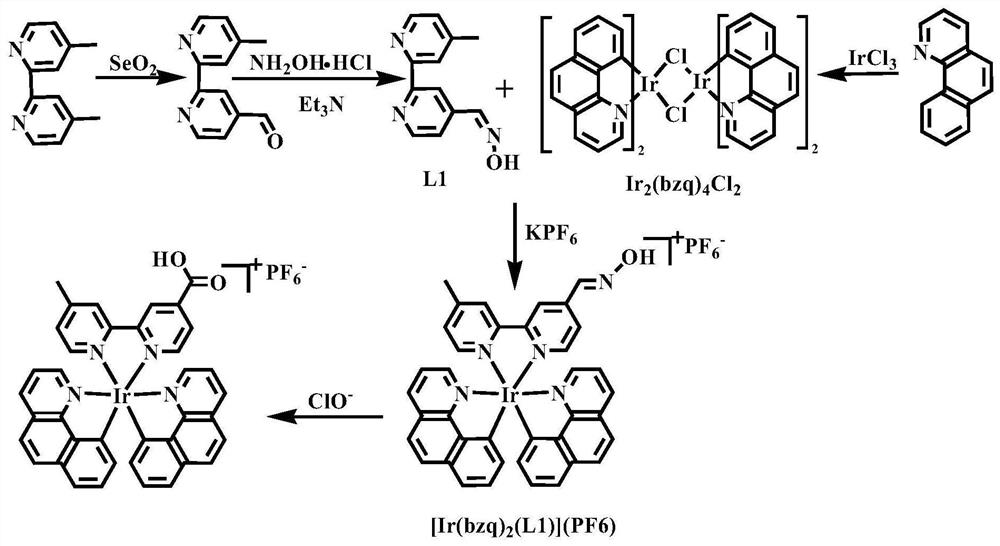
Nitrogen-containing ligand iridium complex as well as preparation method and application thereof
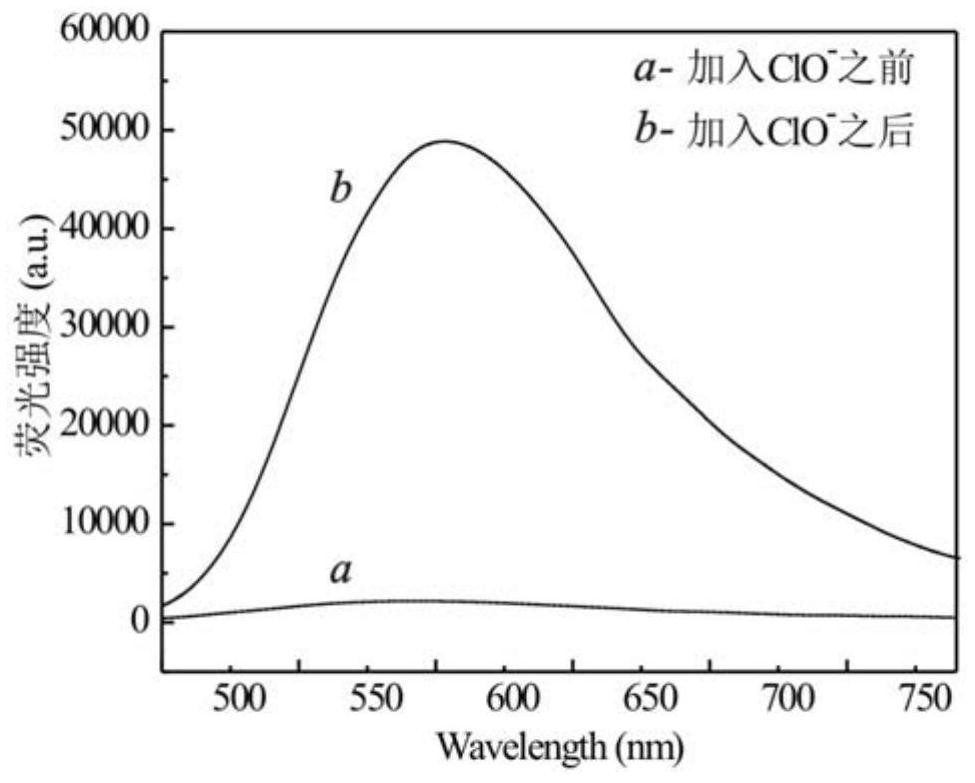
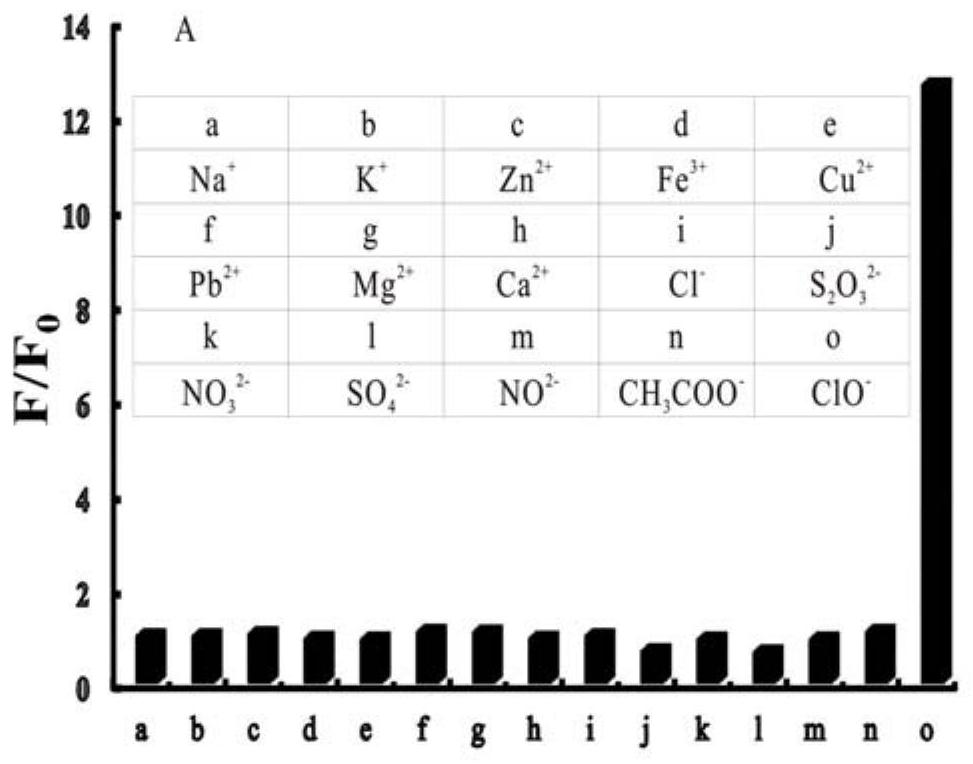
A technology of iridium complexes and nitrogen ligands, applied in indium organic compounds, platinum group organic compounds, chemical instruments and methods, etc., can solve the problem of complex identification and detection methods of hypochlorite, low identification response sensitivity and poor selectivity and other problems, to ensure the identification and detection of hypochlorous acid, broaden the scope of application, and achieve the effect of good conjugation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The preparation method of the iridium complex of the present invention specifically comprises the following steps:
[0042] Step 1, in degassing ethoxyethanol and water (V 脱气乙氧基乙醇 :V 水 =3:1) in the mixed solvent B, add an appropriate amount of iridium trichloride and benzoquinoline (controlling the ratio of the amount of iridium trichloride and benzoquinoline is 2.1~2.3), at 130~170 ℃ Heat to reflux and stir for 10-15 hours, cool to room temperature, and filter under reduced pressure to obtain a brownish-yellow solid, which is washed with a small amount of n-hexane (3ⅹ3mL) and diethyl ether (3ⅹ3mL) successively, and finally dried in a vacuum oven at 40-60°C for 10 ~15h to get the benzoquinoline dimer [Ir 2 (bzq) 4 Cl 2 ] Orange powder product.
[0043] Step 2, take by weighing an appropriate amount of 4,4'-dimethyl-2,2'-bipyridine and selenium dioxide (control 4,4'-dimethyl-2,2'-bipyridine and selenium dioxide The amount ratio is 1~1.1) in a 100mL flask, then add ...
Embodiment 1
[0049] (1) Add 149.3mg iridium trichloride (0.50mmol) and 188.1mg benzoquinoline (1.05mmol) in 9mL of degassed ethoxyethanol and water (v:v=3:1) mixed solvent, in Heated to reflux at 130°C and stirred for 15 hours, cooled to room temperature, and filtered under reduced pressure to obtain a brownish-yellow solid, which was washed with a small amount of n-hexane (3ⅹ3mL) and ether (3ⅹ3mL) successively, and finally dried in a vacuum oven at 40°C for 15h to obtain Benzoquinoline dimer [Ir 2 (bzq) 4 Cl 2 ] orange powder product;
[0050] (2) Weigh 1.8424g 4,4'-dimethyl-2,2'-bipyridine (10.00mmol) and 1.1151g selenium dioxide (10.05mmol) into a 100mL flask, add 50mL1,4-dioxane After the ring, it was heated to reflux at 110°C for 24h. Filtrate while hot to remove precipitated selenium, cool to room temperature, and stand for 1 h, then filter to remove pale yellow 4'-methyl-2,2'-bipyridine-4carboxylic acid precipitate. After removal of the solvent, the residue was redissolved in 5...
Embodiment 2
[0054](1) Add 149.3mg iridium trichloride (0.50mmol) and 197.1mg benzoquinoline (1.10mmol) in 9mL of degassed ethoxyethanol and water (v:v=3:1) mixed solvent, in Heat to reflux at 150°C and stir for 12h, cool to room temperature, filter under reduced pressure to obtain a brownish-yellow solid, wash with a small amount of n-hexane (3ⅹ3mL) and diethyl ether (3ⅹ3mL) successively, and finally use a vacuum oven to dry at 50°C for 12h to obtain Benzoquinoline dimer [Ir 2 (bzq) 4 Cl 2 ] orange powder product;
[0055] (2) Weigh 1.8424g 4,4'-dimethyl-2,2'-bipyridine (10.00mmol) and 1.1096g selenium dioxide (10.00mmol) into a 100mL flask, add 45mL1,4-dioxane After ringing, it was heated to reflux at 100°C for 28h. Filtrate while hot to remove precipitated selenium, cool to room temperature, and stand for 1 h, then filter to remove pale yellow 4'-methyl-2,2'-bipyridine-4carboxylic acid precipitate. After removal of the solvent, the residue was redissolved in 500 mL of ethyl acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com