Preparation method of vinyl ether compound
A technology of vinyl ether and benzyloxy vinyl ether, which is applied in the field of preparation of vinyl ether compounds, can solve problems such as high temperature reaction, complicated operation, and danger of acetylene gas explosion, and achieve high yield, simple operation, good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
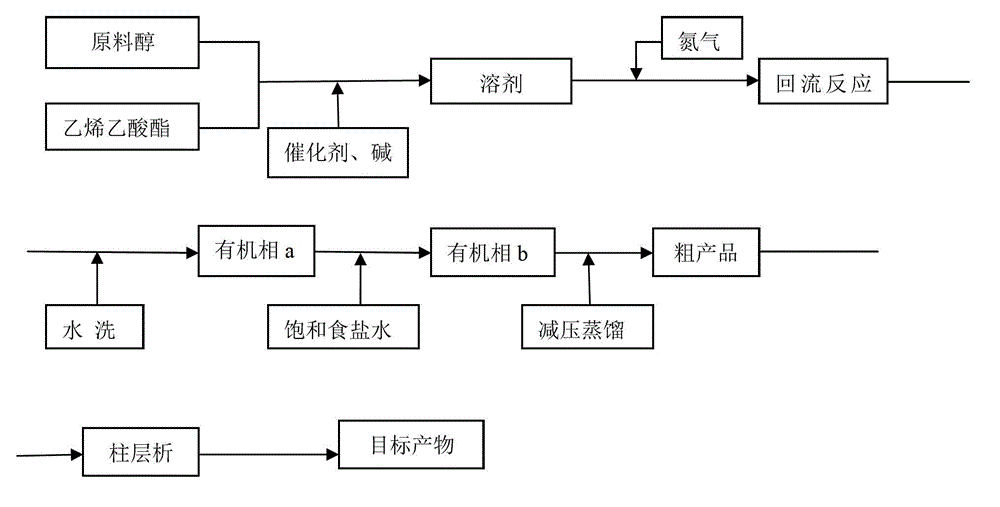
[0035] A kind of preparation method of vinyl ether compound, described vinyl ether compound is benzyloxy vinyl ether or triethylene glycol divinyl ether, combining figure 1 , figure 2 , the method for preparing the vinyl ether compound comprises the following steps:
[0036] The first step, the preparation of catalyst, specifically comprises the following steps:
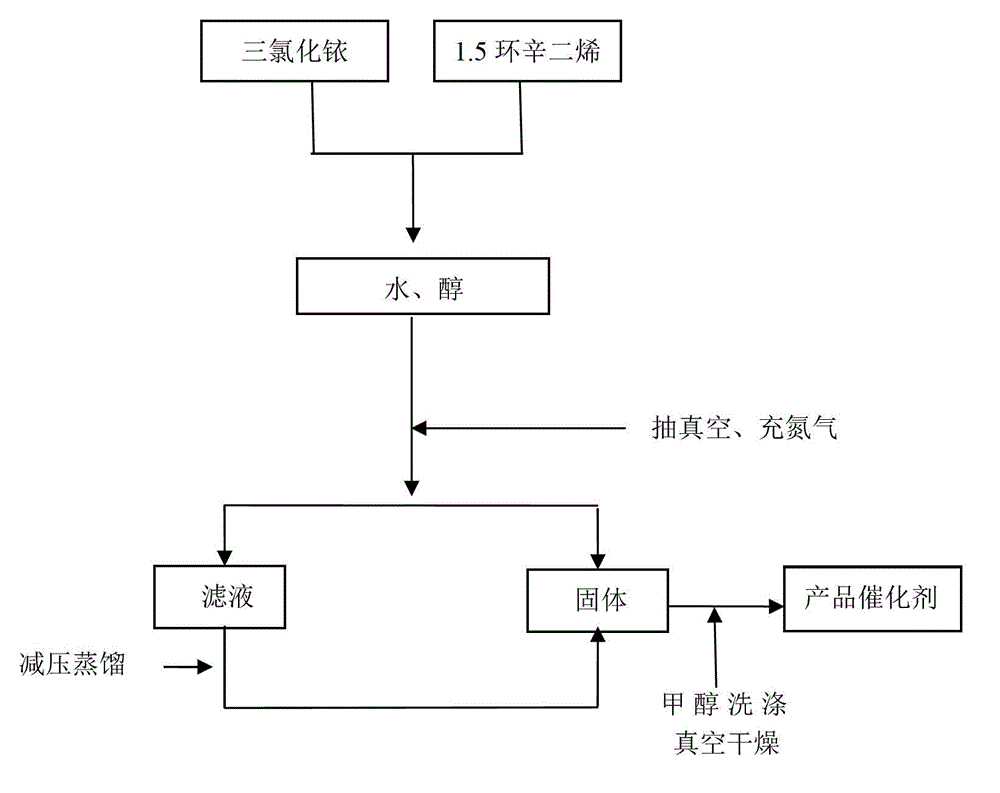
[0037] 1) adding iridium trichloride and 1,5-cyclooctadiene to the reaction device, then adding a mixture of water and alcohol to the reaction device, stirring evenly to obtain a reaction solution;
[0038] 2) After sealing the reaction device, vacuumize and fill with nitrogen, so that the reaction solution is heated and refluxed in a nitrogen atmosphere to react;
[0039] 3) After the reaction is finished, the reaction solution is cooled to room temperature and filtered, the filter residue is washed with methanol and then vacuum-dried, and the solid obtained after the filtrate is distilled under reduced pressure ...
Embodiment 1
[0047] Embodiment 1: Taking benzyloxy vinyl ether as an example, the chemical reaction equation is as follows:
[0048]
[0049] combine figure 1 , figure 2 , the preparation method of vinyl ether compound benzyloxy vinyl ether of the present invention, comprises the following steps:
[0050] The first step, catalyst [Ir(COD)Cl] 2 Preparation, the reaction equation is as follows:
[0051]
[0052] Specifically include the following steps:
[0053] 1) Add 0.1g (0.00028mol) of iridium trichloride and 0.12mL (0.001mol) of 1,5 cyclooctadiene to the reaction device, then add 3.5mL (0.194mol) of water and 1.6 mL (0.021mol) mixture of isopropanol, stirred evenly to obtain reaction solution;
[0054] 2) After sealing the reaction device, evacuate it, fill it with nitrogen, and heat the reaction solution at 80-100°C in an atmosphere of nitrogen to reflux for 10-14 hours;
[0055] 3) After the reaction is over, the reaction solution is cooled to room temperature and filtere...
Embodiment 2
[0065] Embodiment 2: reaction step is identical with embodiment 1, difference is:
[0066] Change the volume of 1,5-cyclooctadiene used in the reaction in the first step 1) to 0.067mL (0.00056mol), 0.15mL (0.00125mol), 0.21mL (0.0017mol) respectively, and keep the rest of the conditions unchanged. Catalyst [Ir(COD)Cl] in the first step 2 The yields were 65.1%, 77.1% and 78.2%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com