Method for preparing 4-amino benzene boric acid hydrochloride
A technology of aminophenylboronic acid hydrochloride and nitrophenylboronic acid, which is applied in the field of preparing 4-aminophenylboronic acid hydrochloride, can solve the problems of cumbersome operation and harsh conditions, and achieve safe and reliable process, mild conditions and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A method for preparing 4-aminophenyl borate hydrochloride with biscatechol borate:
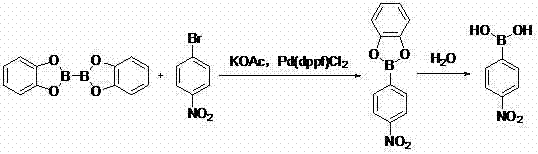
[0020] In the first step, add 500.0g DMF, 101.0g 4-nitrobromobenzene (0.50mol, 1eq), 130.8g bis-catechol borate (0.55 mol, 1.2eq), 147.2g potassium acetate (1.5mol, 3eq), 18.3gPd(dppf)Cl 2 (0.025mol, 0.05eq), react at 80°C for 3h. Cool down to 20~25°C, filter, add the filtrate dropwise to 500.0g of water, stir for 1 hour, then raise the temperature to 20~25°C. Extracted twice with ethyl acetate (200mL×2), combined the organic layers and concentrated, slurred and filtered with 50.0g n-heptane at -5~0°C, and dried the filter cake to obtain 63.7g of white solid, GC: 97.6%, yield: 76.3 %.
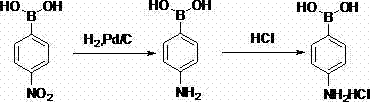
[0021] In the second step, dissolve 63.7g of 4-nitrophenylboronic acid (0.38mol, 1eq) into 255g of ethyl acetate, add 2.0g of palladium carbon (10%, 0.005eq), hydrogenate at 0.8~1.0MPa, 70~80℃ React for 6h, after the reaction is over, filter with diatomaceous earth, cool the filtrate to 0°C, add 51.0...
Embodiment 2
[0023] A method for preparing 4-aminophenyl borate hydrochloride with biscatechol borate:
[0024] In the first step, add 500.0g DMSO, 101.0g 4-nitrobromobenzene (0.50mol, 1eq), 130.8g bis-catechol borate (0.55 mol, 1.2eq), 147.2g potassium acetate (1.5mol, 3eq), 18.3gPd(dppf)Cl 2 (0.025mol, 0.05eq), react at 90°C for 2.5h. Cool down to 20~25°C, filter, add the filtrate dropwise to 500.0g of water, stir for 1 hour, then raise the temperature to 20~25°C. Extracted twice with ethyl acetate (200 mL×2). The combined organic layers were concentrated, slurred and filtered with 50.0 g of n-heptane at -5~0°C, and the filter cake was dried to obtain 65.6 g of white solid, GC: 97.1%, yield: 78.6%.
[0025] In the second step, dissolve 65.6g of 4-nitrophenylboronic acid (0.39mol, 1eq) into 262g of ethyl acetate, add 2.1g of palladium carbon (10%, 0.005eq), hydrogenate at 0.8~1.0MPa, 70~80℃ React for 6 hours, the reaction is over, filter with diatomaceous earth, cool the filtrate to 0...
Embodiment 3
[0027] A method for preparing 4-aminophenyl borate hydrochloride with biscatechol borate:
[0028] In the first step, add 500.0g of 1,4-dioxane, 101.0g of 4-nitrobromobenzene (0.50mol, 1eq), and 130.8g of bis-phthalate to a magnetically stirred 1L four-neck flask under argon protection. Diphenol borate (0.55mol, 1.2eq), 147.2g potassium acetate (1.5mol, 3eq), 18.3gPd(dppf)Cl 2 (0.025mol, 0.05eq), react at 100°C for 2h. Cool down to 20~25°C, filter, add the filtrate dropwise to 500.0g of water, stir for 1 hour, then raise the temperature to 20~25°C. Extracted twice with ethyl acetate (200mL×2), the combined organic layers were concentrated, 50.0g of n-heptane was used to beat and filter at -5~0°C, and the filter cake was dried to obtain 60.4g of white solid, GC: 98.3%, yield: 72.4 %.
[0029] In the second step, dissolve 60.4g of 4-nitrophenylboronic acid (0.36mol, 1eq) into 241g of ethyl acetate, add 2.0g of palladium carbon (10%, 0.005eq), hydrogenate at 0.8~1.0MPa, 70~80℃...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com