Phosphine-containing aromatic diamine compound, preparation method and application thereof
A technology of aromatic diacids and compounds, which is applied in the field of phosphine-containing aromatic diamine compounds and their preparation and application, can solve the problems of poor film transparency and low heat resistance of polyimide, and achieve improved heat resistance, The effect of high glass transition temperature and good visible light transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、3
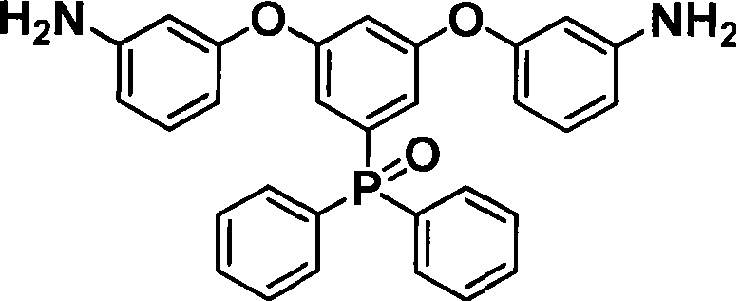
[0029] Embodiment 1,3, the synthesis of 5-BADPO
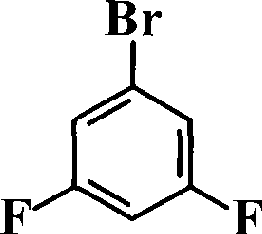
[0030] Add 0.165mol (4.0095g) of magnesium powder and 27ml of tetrahydrofuran into a 500ml three-neck flask, and use an ice-water bath to control the temperature of the reactants in the reaction flask at 0-5°C. Mix 0.15mol (28.9485g) of 3,5-difluorobromobenzene and 68ml of tetrahydrofuran in a constant pressure funnel, and slowly drop them into the system under nitrogen protection. After all the drops were completed, the temperature of the reaction system was slowly returned to room temperature, and stirring was continued for 3 h to obtain a gray-black solution.
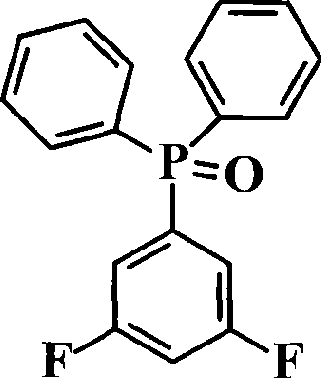
[0031]Cool the temperature of the reaction system to 0-5°C with an ice-water bath; add 0.1254mol (29.6863g) of diphenylphosphoryl chloride and 41ml of tetrahydrofuran into a constant pressure funnel, and then slowly drop them into the reaction system. After the drop, the temperature of the reaction system was slowly returned to room temperature, and the stirring was ...
Embodiment 2、3
[0044] Embodiment 2,3, the synthesis of 5-BADPO
[0045] The synthesis of 3,5-difluorophenyldiphenylphosphine oxide is the same as in Example 1.
[0046] In a 250ml three-necked flask, add 0.02mol (6.2854g) 3,5-difluorophenyl diphenylphosphine oxide, 0.042mol (4.5835g) 3-aminophenol, 0.052mol (7.1869g) anhydrous potassium carbonate, 35ml 1 , 3-dimethylimidazolinone (DMI) and 18.4ml toluene. Heated to 135°C under nitrogen protection, and maintained constant temperature for 12h. Part of the toluene was distilled off, the temperature of the system was raised to 190° C., and the stirring was continued for 3 h to obtain a black solution. Cool to room temperature, filter to obtain a brown solution, pour into 5% acetic acid aqueous solution, and mechanically stir to obtain a brown precipitate. The precipitate was filtered out, dried under vacuum at 65°C, recrystallized with ethanol, and decolorized with activated carbon. A light brown solid was obtained, and the yield of the seco...
Embodiment 3、3
[0052] Embodiment 3, 3, the synthesis of 5-BADPO
[0053] The synthesis of 3,5-difluorophenyldiphenylphosphine oxide is the same as in Example 1.
[0054] Add 0.02mol (6.2854g) 3,5-difluorophenyldiphenylphosphine oxide, 0.042mol (4.5835g) 3-aminophenol, 0.052mol (7.1869g) anhydrous potassium carbonate, 35ml in a 250ml three-necked flask, N,N-Dimethylacetamide (DMAc), and 18.4 ml toluene. Heated to 135°C under the protection of nitrogen, and maintained constant temperature reaction for 15h. Part of the toluene was distilled off, the temperature of the system was raised to 160°C, and the stirring was continued for 4 hours to obtain a black solution. Cool to room temperature, filter to obtain a brown solution, pour into 5% acetic acid aqueous solution, and mechanically stir to obtain a brown precipitate. The precipitate was filtered out, dried under vacuum at 65°C, recrystallized with ethanol, and decolorized with activated carbon. A light brown solid was obtained with a seco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com