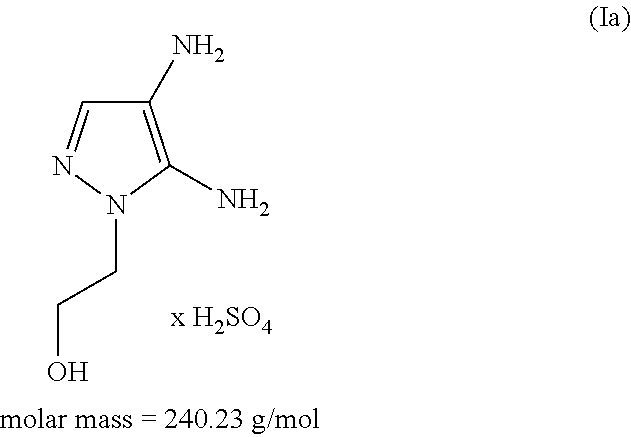
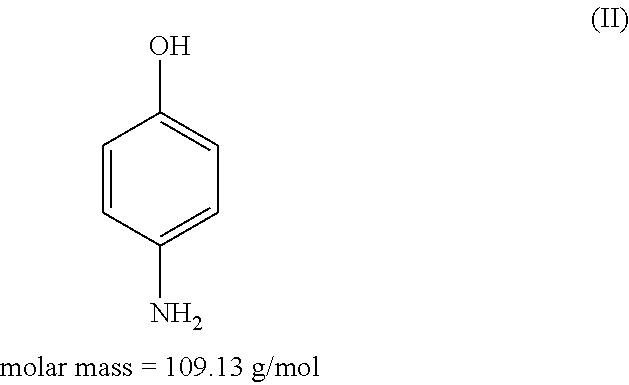
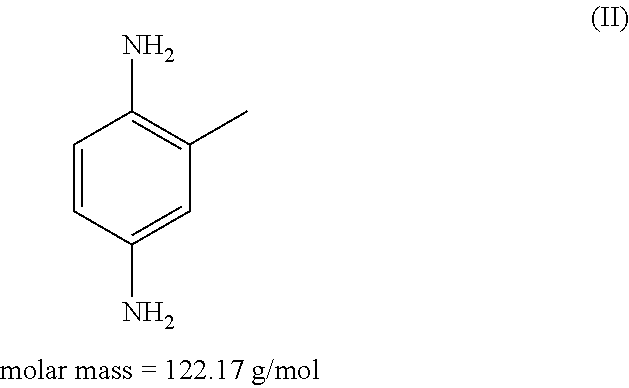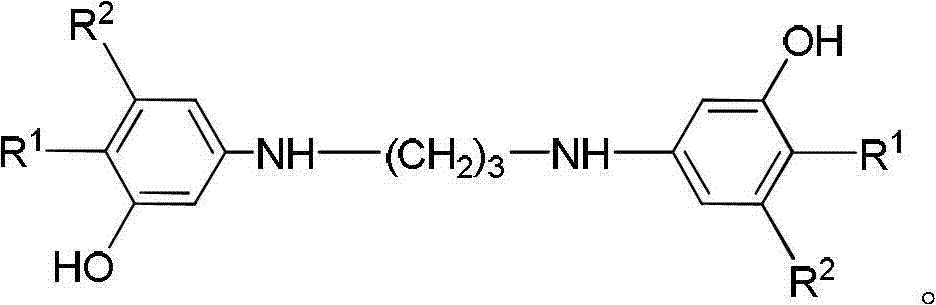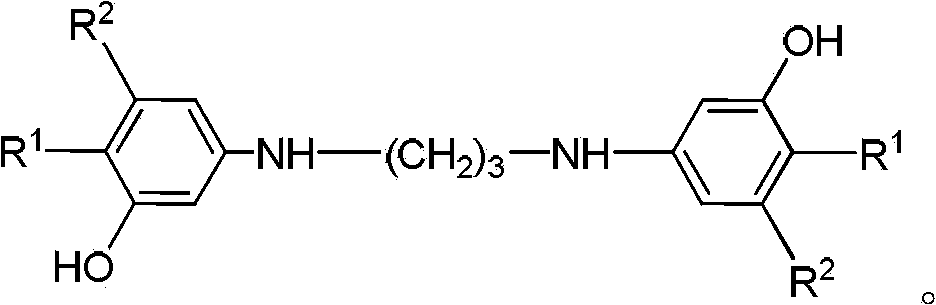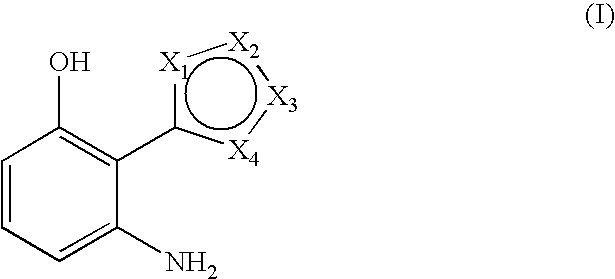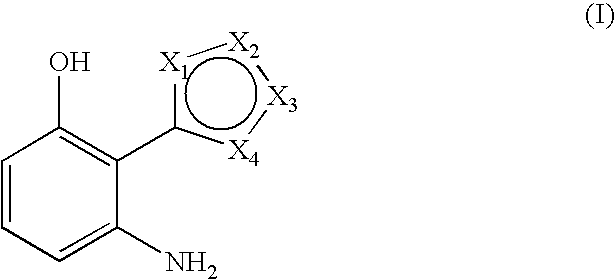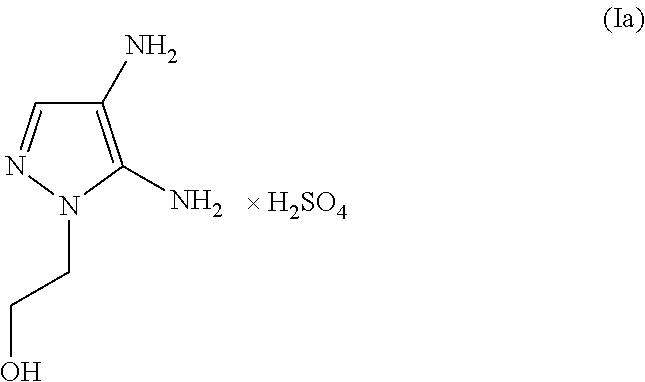Patents
Literature
33 results about "3-Aminophenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
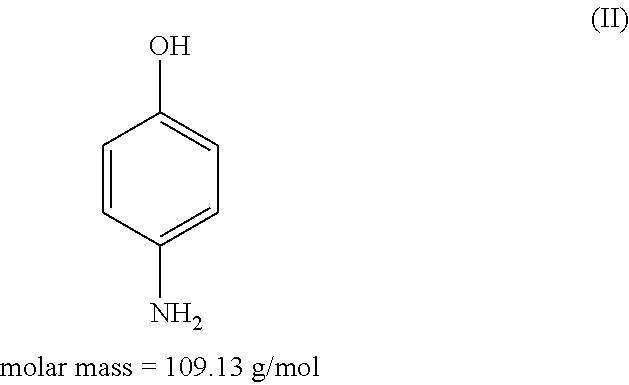
3-Aminophenol is an organic compound with formula C₆H₄(NH₂)(OH). It is an aromatic amine and aromatic alcohol. It is the meta isomer of 2-aminophenol and 4-aminophenol.
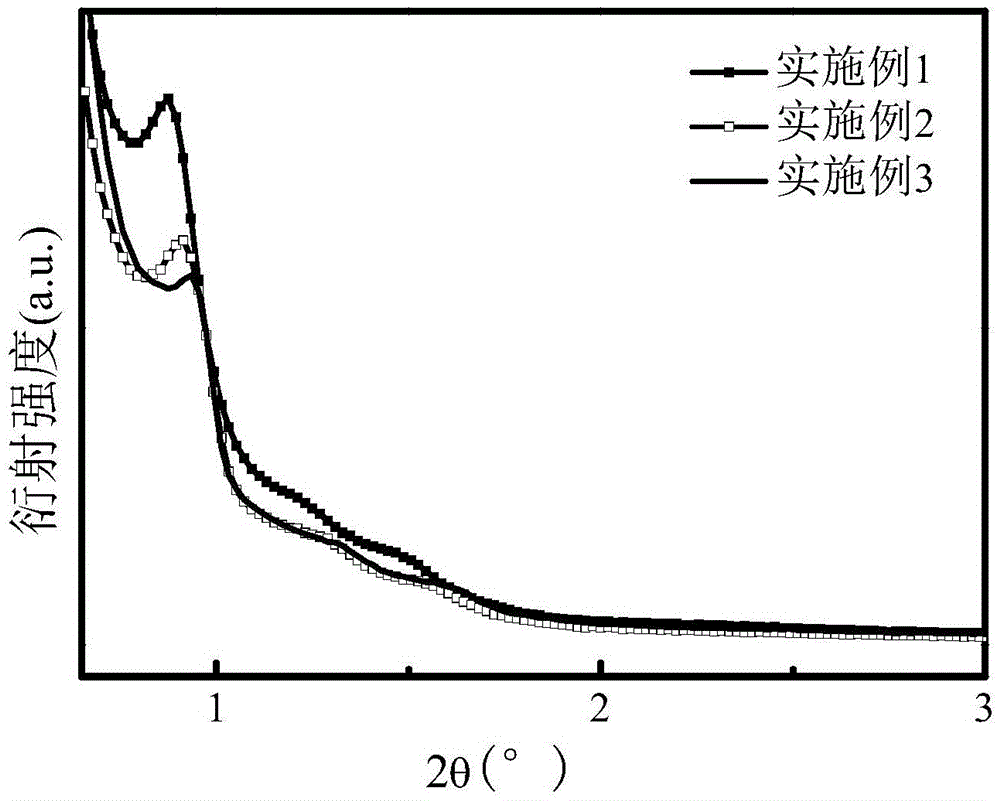
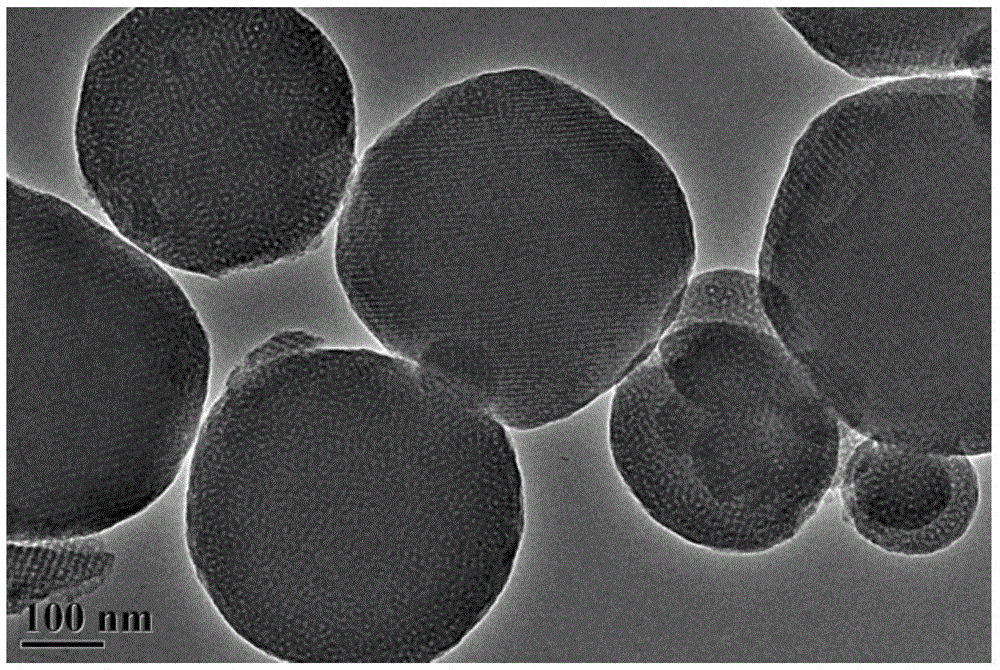
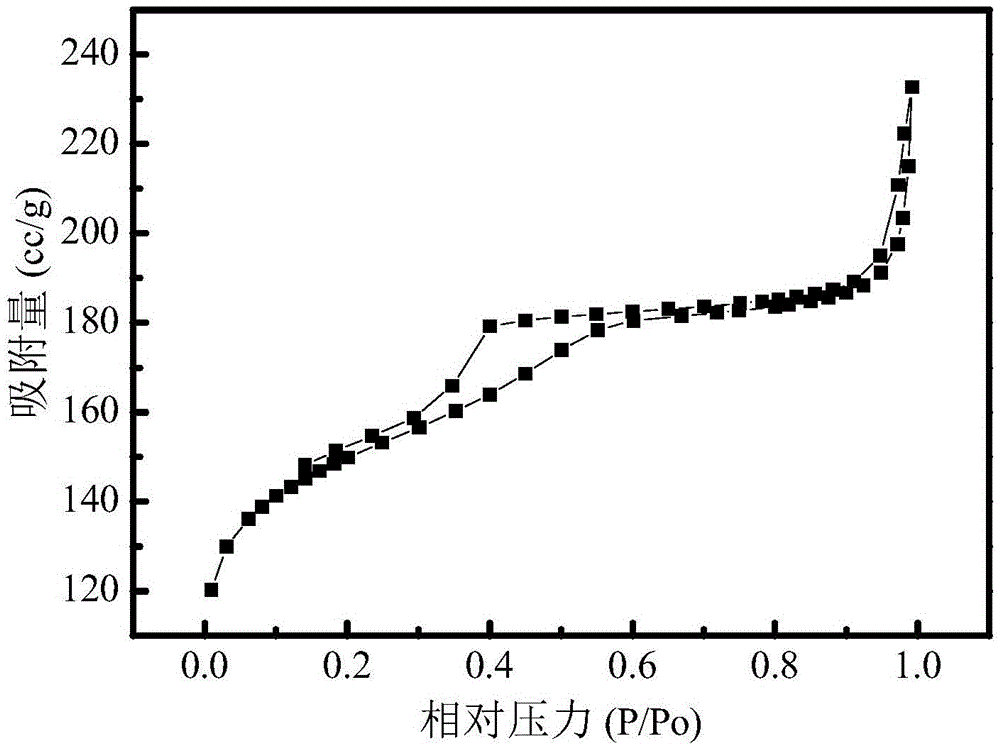
Preparation method of nitrogen and sulfur co-doping micropore-mesopore carbon microspheres
InactiveCN106492749ALarge specific surface areaRich mesostructureOther chemical processesWater contaminantsMicrosphereCarbonization
The invention relates to a preparation method of nitrogen and sulfur co-doping micropore-mesopore carbon microspheres, and belongs to the scientific and technical field of materials. The method comprises the steps that 3-aminophenol, a formaldehyde solution, an L-cysteine, a surface active agent, silica soil, ethyl alcohol and water are taken on the basis of the mass ratio being 1:(1.2-1.8):(0.5-1.5):(0.5-1.5):(1-3):(32-82):(40-100); the water and ethyl alcohol are mixed to be uniform under the temperature of 20-35 DEG C, and the other raw materials are added into the mixture in sequence, the reaction is performed for 24 h, hydrothermal treatment is performed under the temperature of 100 DEG C for 24 h, under the nitrogen atmosphere, the room temperature is increased to 600-900 DEG C at the heating rate of 2-10 DEG C / min for carbonization, NaOH is used for removing SiO2, and nitrogen and sulfur co-doping mesoporous carbon microspheres are obtained. The obtained carbon microspheres, KOH and water are taken on the basis of the mass ratio being 1:(1-4):(20-50) and mixed to be uniform, after drying, in the nitrogen atmosphere, the room temperature is increased to 600-900 DEG C at the heating speed of 2-10 DEG C / min for activation, and the nitrogen and sulfur co-doping micropore-mesopore carbon microspheres are obtained. The technology is simple, the obtained nitrogen and sulfur co-doping micropore-mesopore carbon microspheres are wide in application prospect on the aspect of adsorption and removal of heavy metal ions in wastewater.
Owner:TONGJI UNIV
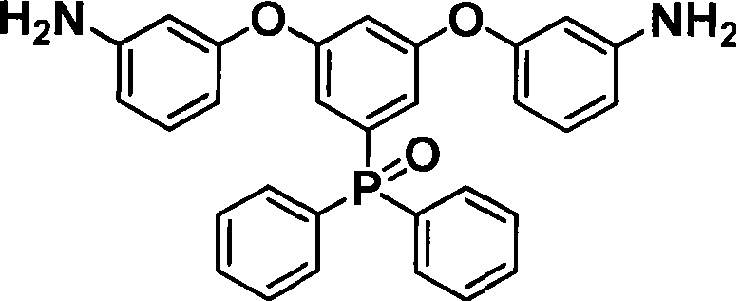
Phosphine-containing aromatic diamine compound, preparation method and application thereof
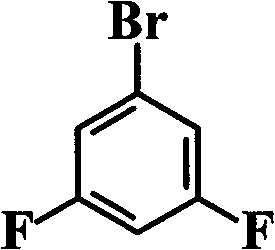
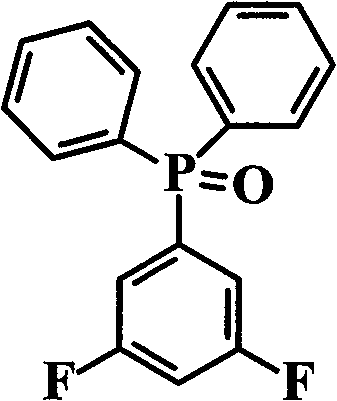
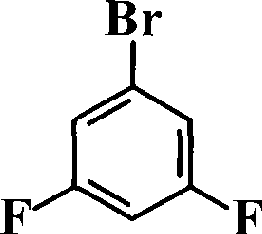
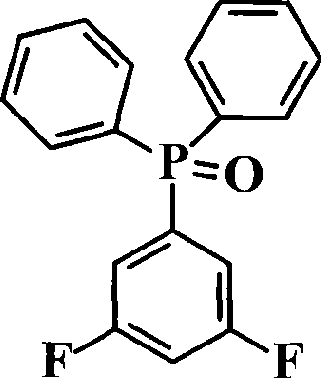
ActiveCN101531678AImprove heat resistanceHigh glass transition temperatureGroup 5/15 element organic compoundsGrignard reactionPhosphine oxide
The invention discloses an aromatic diamine compound containing aryl phosphine groups, a prepration method and an application thereof. The structural general formula of the compound is shown in a formula I. The compound takes 3,5-difluoro-bromobenzene and diphenylphosphinic chloride as raw materials, firstly prepares 3,5-difluorophenyl diphenyl phosphine oxide by Grignard reaction and then continuously reacts with 3-aminophenol for preparation. The compound has smaller steric effect and has great value for improving the heat resistance performance of atomic oxygen resistant polyimide thin film materials and improving the visible light transparency. In addition, polyimide phosphine-containing polymer materials prepared by utilizing the phosphine-containing aromatic diamine compound have good atomic oxygen resistance performance, higher glass transition temperature and better visible light transparency, thereby being capable of being applied in the manufacturing of satellites and other aircraft parts in the LEO environment.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Trichoderma pseudokoningii exocellular polysaccharide, preparation method and application thereof
InactiveCN101220101AImprove immune activityEnhance phagocytic activityOrganic active ingredientsMicroorganism based processesMethod testMonosaccharide composition
The invention discloses a polysaccharide from Trichoderma pseudokoningii, which is characterized in that (1) the high performance gel filtration chromatography test shows that the polysaccharide has a symmetrical peak with 18325 molecular weight; (2) an improved sulfuric acid-phenol method and a 3-aminophenol method test show that the neutral polysaccharide content is 65.2 percent, while the uronic acid content is 32.6 percent; (3) the GC analysis of the alditol acetate shows that the monosaccharide components are rhamnose, glucose and galactose, the molar ratio is Rha:Glc:Gal relates to 5.6:2.7:1.0, while when the polysaccharide is completely reduced, the molar ratio is Rha:Glc:Gal relates to 14.5:9.3: 1.0, having 26.6 molar percent glucuronic acid. The preparation method of the polysaccharide from Trichoderma pseudokoningii consists of the crude polysaccharide preparation and the polysaccharide purification. The polysaccharide from the Trichoderma pseudokoningii has broad applications in preparing the drugs or functional food for improving the mammal immunocompetence and for treating or adjuvant treating the tumors.
Owner:SHANDONG UNIV
Fluorescent probe for detecting hydrogen peroxide and preparation method and application thereof
ActiveCN105647518AGood choiceImprove universalityOrganic chemistryMethine/polymethine dyesFluorescenceSynthesis methods
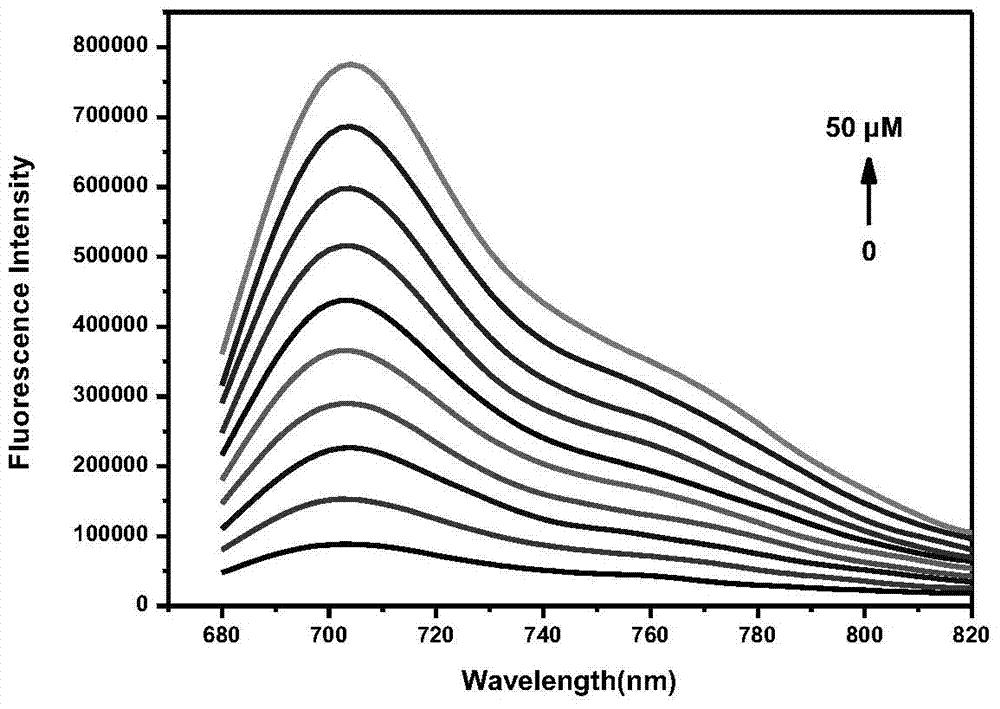
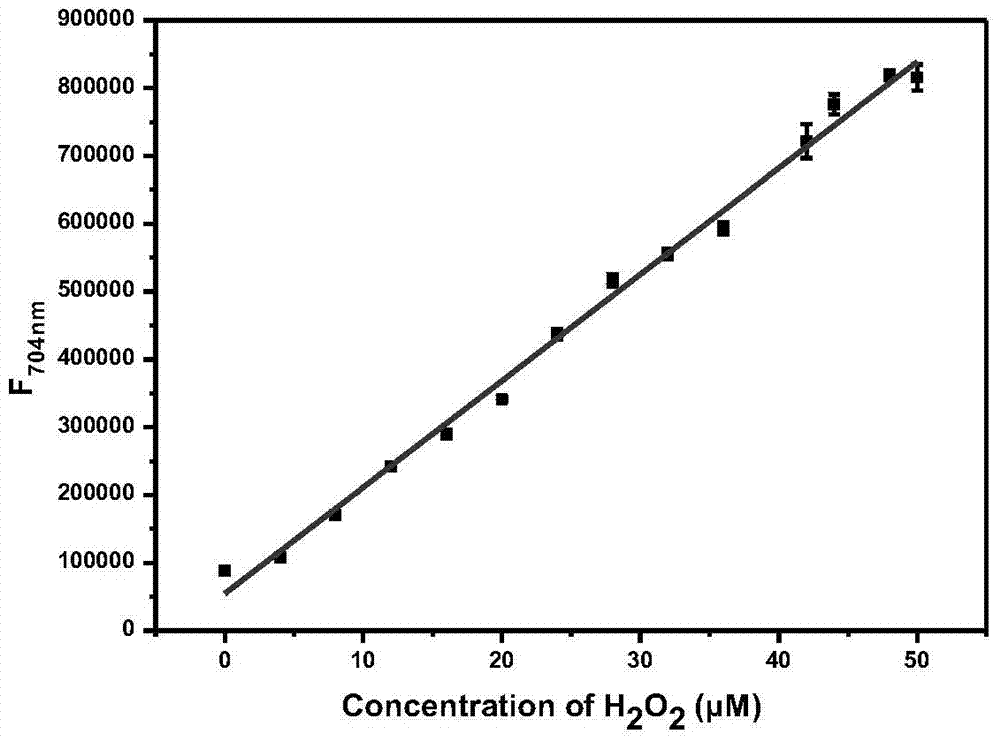
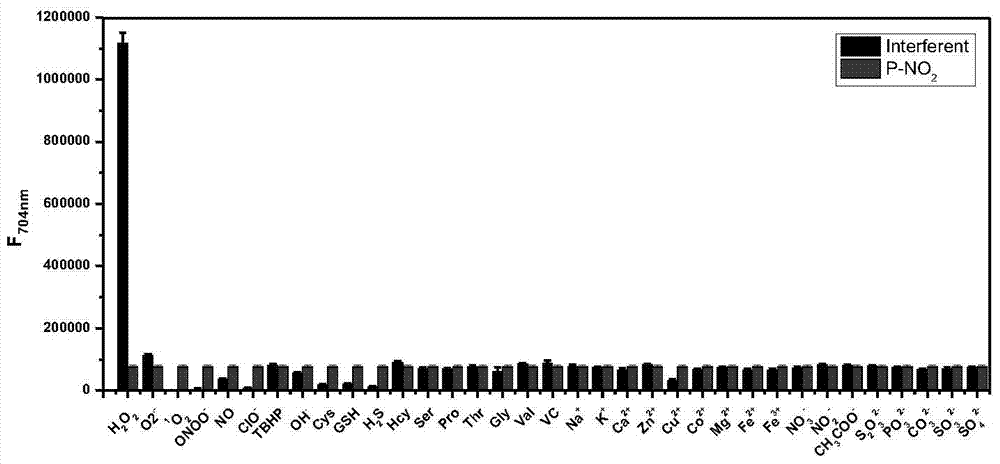
The invention discloses a fluorescent probe for detecting hydrogen peroxide and a preparation method and application thereof. The structural formula of the fluorescent probe for detecting hydrogen peroxide is shown in the description, wherein any one or two of R1-R5 represents electron withdrawing groups. The fluorescent probe is novel in group response, good in selectivity and high in sensitivity; the excitation emission wavelength of the fluorescent probe is located in the range of near infrared waves, damage to tissue is small, the penetrating power is high, and the fluorescent probe can be used for living body imaging. The preparation method comprises the steps that 3-aminophenol and Cy.7.Cl are subjected to a substitution reaction, and Cy-Ph-NH2 is obtained; a retro-Knoevenagel reaction is conducted, and P-NH2 is obtained; P-NH2 and a derivative of phenylglyoxylic acid are subjected to the substitution reaction, and the product P-R is obtained, that is, the fluorescent probe for detecting hydrogen peroxide is obtained. The synthesizing method is simple and suitable for industrial production.
Owner:SHANDONG NORMAL UNIV
Method for preparing mono-dispersed N-doped ordered mesoporous carbon spheres with particle size of 100-800 nm at high yield in single-pass mode
ActiveCN105565296AIncrease concentrationAchieve outputMaterial nanotechnologyCarbon preparation/purificationAlcoholSorbent
The invention discloses a method for preparing mono-dispersed N-doped ordered mesoporous carbon spheres with the particle size of 100-800 nm at high yield in a single-pass mode. According to the method, F127, 3-aminophenol and NH3.H2O serve as a template, a carbon and nitrogen source and a catalyst respectively, and the mono-dispersed N-doped ordered mesoporous carbon spheres with the particle size of 100-800 nm are produced at high yield in the single-pass mode on a large scale by regulating and controlling product morphology through ethyl alcohol. The preparing method is easy to implement, the concentration of the carbon and nitrogen source in the system is high, dosage of solvent is greatly reduced, several grams of N-doped ordered mesoporous carbon spheres can be obtained at high yield in the single-pass mode, the obtained N-doped ordered mesoporous carbon spheres are small in size distribution range, good in monodispersity and regular in pore structure and can be directly used as a catalyst, adsorbent and the like.
Owner:SHAANXI NORMAL UNIV +1
Dye composition comprising at least one oxidation base, 2-chloro-6-methyl-3-aminophenol and 3-methyl-1-phenyl-5-pyrazolone
Provided is a dye composition comprising at least one of oxidation base, 2-chloro-6-methyl-3-aminophenol and 3-methyl-1-phenyl-5-pyrazolone. The composition is used for dyeing keratin fiber, specifically human keratin fiber. Also, provided is the use of 3-methyl-1-phenyl-5-pyrazolone as a stabilizer of 2-chloro-6-methyl-3-aminophenol.
Owner:LOREAL SA
Preparation method of hollow nitrogen-doped carbon nanotubes
PendingCN109987596AShape stableLong diameter heightMaterial nanotechnologyCarbon nanotubesCarbonizationMetallic materials
The invention belongs to the field of inorganic non-metal materials, and particularly relates to a preparation method of hollow nitrogen-doped carbon nanotubes. According to the preparation method, asilicon dioxide template is prepared by using tetraethyl orthosilicate (TEOS) as a silicon dioxide precursor, 3-amino phenol formaldehyde resin (APF) is used as carbon source and a nitrogen source, aconcentration of a nickel chloride solution, addition amounts of 3-aminophenol and formaldehyde and carbonization temperature are adjusted to realize control of a length-diameter ratio, a nitrogen content and structural parameters of a Ni@N doped C composite material, and after etching is performed to remove silicon, the hollow nitrogen-doped carbon nanotubes with a high N content, a high specificsurface area, a large pore volume, the adjustable length-diameter ratio and a mesoporous size can be finally prepared.
Owner:CHANGZHOU UNIV
Preparation method of catalyst for H2S selective catalytic oxidation
InactiveCN107983392AThe synthesis method is simpleUniversalGas treatmentPhysical/chemical process catalystsTetramineCarbonization
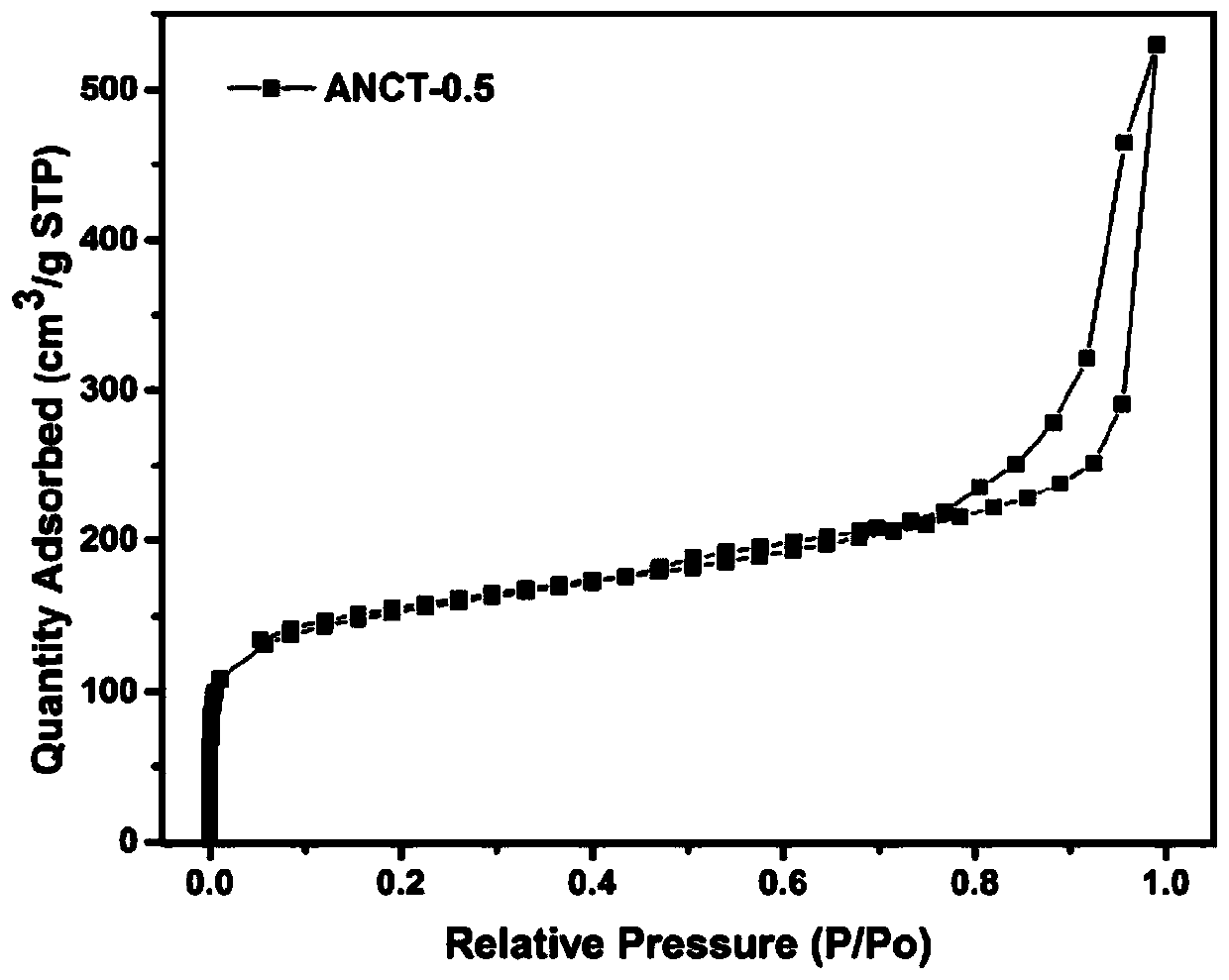
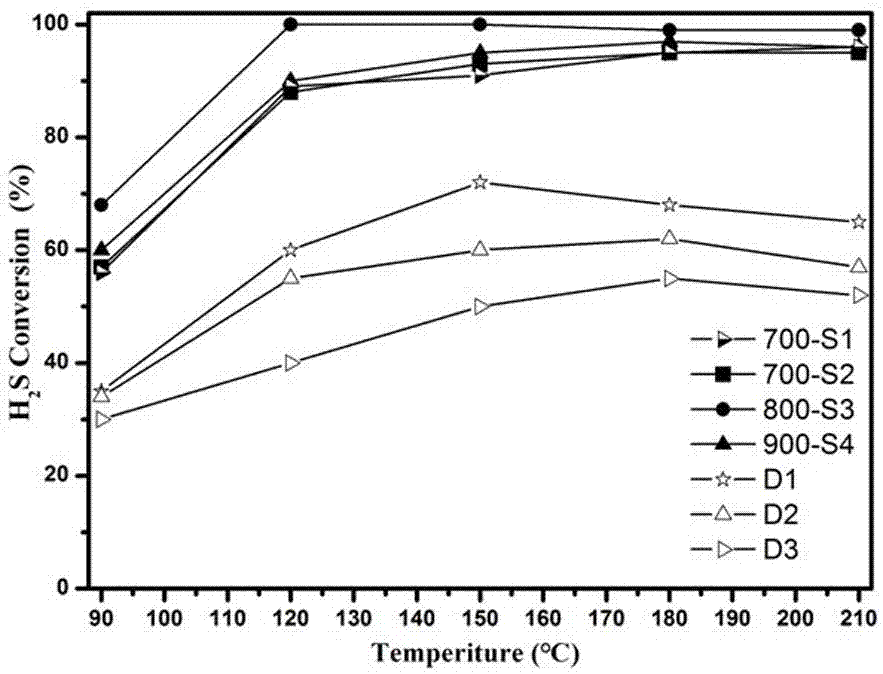
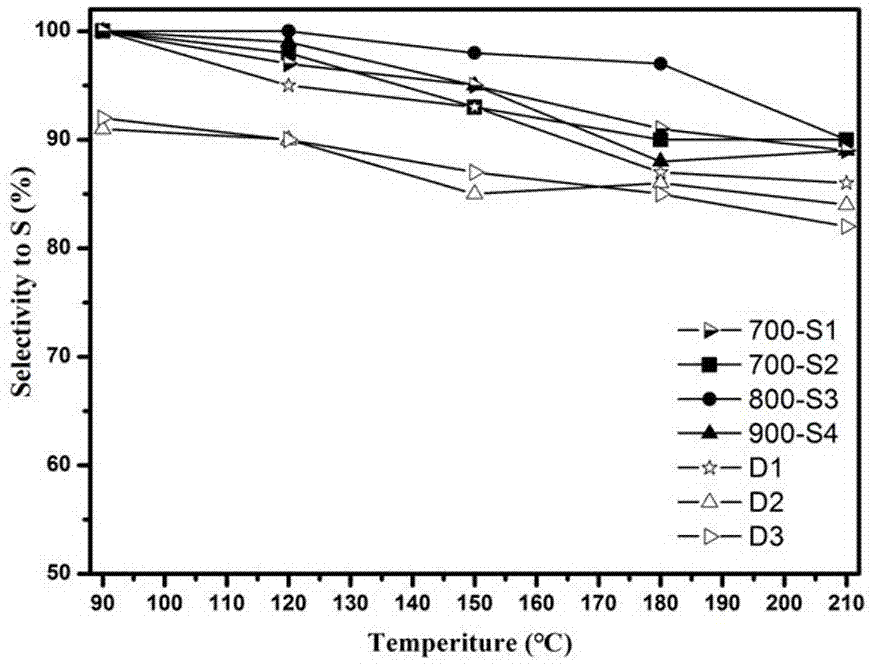
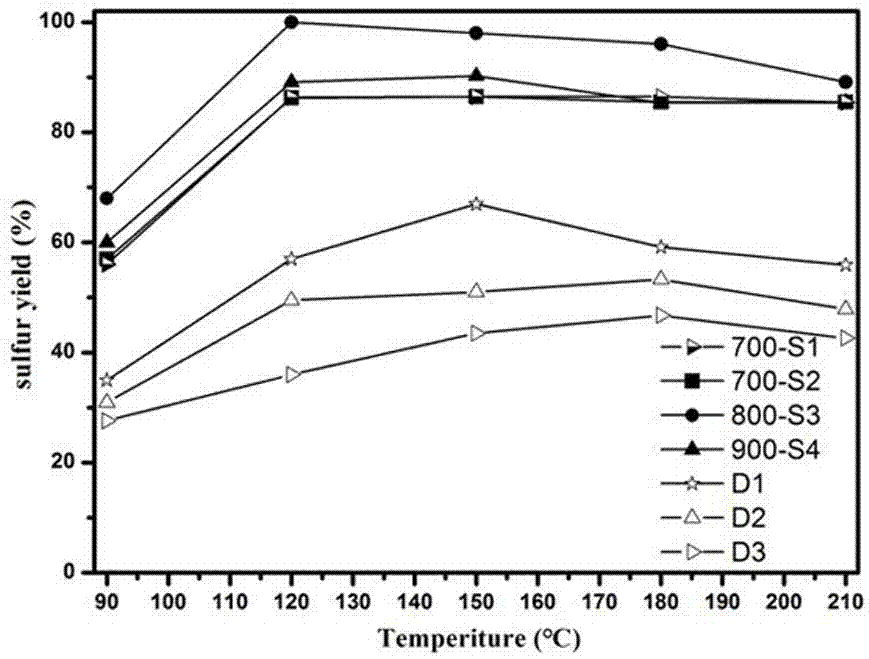
The invention discloses a preparation method of a catalyst for H2S selective catalytic oxidation. The catalyst is prepared by the following steps: taking F217 as the template and 3-aminophenol and hexamethylene tetramine as the precursors, carrying out rapid carbonization in a nitrogen atmosphere, and finally performing KOH etching. The prepared catalyst has the advantages of large specific surface area and high N-doping amount. The prepared sample can be use as a catalyst applied to H2S selective catalytic oxidation. Compared with a common H2S selective catalyst, the prepared catalyst has a strong H2S selective catalytic performance.
Owner:FUZHOU UNIV
Method for making 8-hydroxyjulolidine compound
InactiveUS20020120142A1Reduce manufacturing costIncrease productionOrganic chemistryAcetoacetatesAcetic acid
The present invention relates to 8-hydroxyjulolidine, its analogous compounds and their preparation methods. The invented method comprises one single step of cyclization reactions of 3-aminophenol or 1,3-dihydroxyaniline with 1,3-dihalopropane or its analogs, to prepare the desired julolidines with improved yields. The products of this invention have the following structures: wherein R1 and R2 represent independently H, halogen, hydroxyl or alkyl. The method of this invention includes the following chemical reaction: wherein X and Y represent independently halogen, acyloxyl, sulfonyloxyl or phosphoryloxyl and R1 and R2 are as defined above. This invention also discloses method for the preparation of various coumarins by additionally reacting the produced 8-hydroxyjulolidine and its analogs with appropriate reagents such as malonate and acetoacetate. This invention also discloses the products and intermediates prepared therefrom.
Owner:ALLIED IND
Method for preparing paper-base mesoporous carbon electrode material and method for preparing electrode
InactiveCN108455561ALarge specific surface areaHigh specific capacitanceHybrid capacitor electrodesCarbon preparation/purificationActivation methodCarbon composites
The invention provides a method for preparing a paper-base mesoporous carbon electrode material and a method for preparing an electrode, and belongs to the field of carbon material preparation methods. The method for preparing the paper-base mesoporous carbon electrode material comprises the steps that a certain amount of industrial filter paper, F127, 3-aminophenol, hexamethylenetetramine, trimethylbenzene and water are weighed, mixed, evenly stirred and transferred into a reaction still for reaction and cleaning, then placed in a tube furnace after being dried for carbonization in the nitrogen atmosphere, and finally cooled to the indoor temperature, 0.1 Mol of hydrogen chloride and deionized water are used for washing the mixture to the neutral, and drying is carried out; then a certainmass ratio of sample is weighed, after potassium hydroxide activation is carried out, the mixture is taken out after being dried in a drying box, and then the material is obtained. According to the method for preparing the paper-base mesoporous carbon electrode material and the method for preparing the electrode, a layer of carbon-carbon compound material of mesoporous carbon microspheres is loaded on the surface of the material through a one-step hydrothermal method, nitrogen, oxygen and other heteroatoms exist on the surface of the material, so that the material has good wettability and better ion accessibility. The potassium hydroxide activation method makes the specific surface area and pore structure of the material both have obvious improvement, the specific capacity is high, and the cycle performance is good.
Owner:NORTHEAST FORESTRY UNIVERSITY
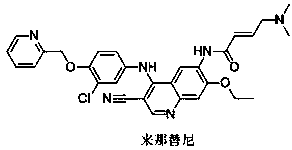
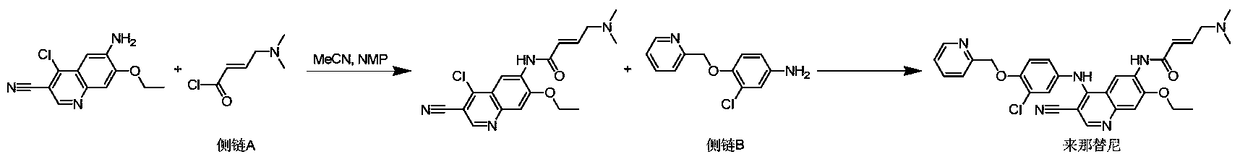
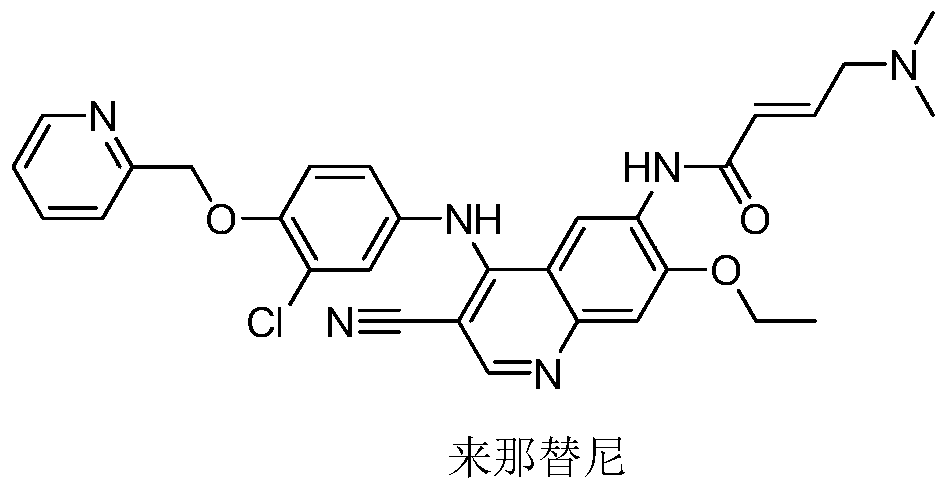
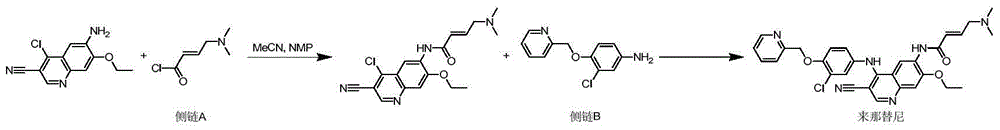
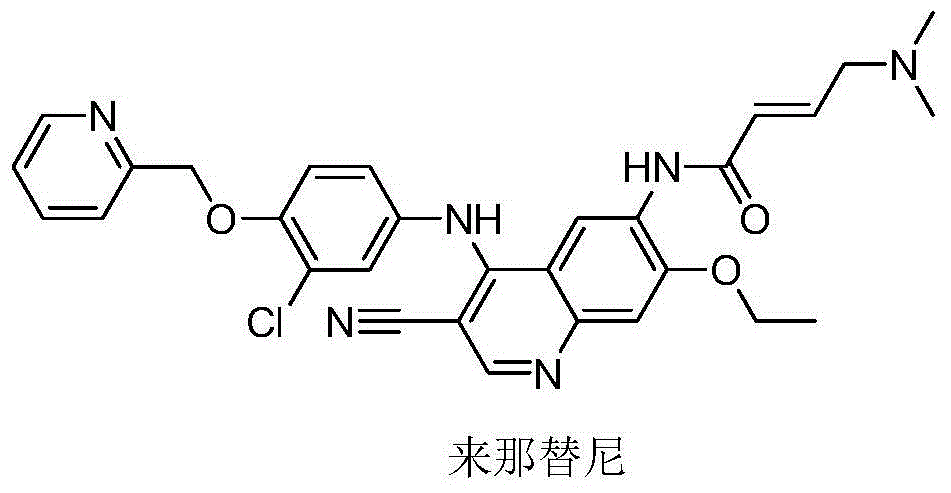
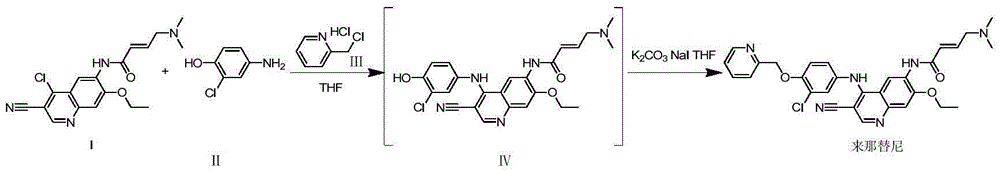
Novel preparation method of epidermal growth factor receptor (EGFR) inhibitor neratinib
The invention relates to a preparation method of an epidermal growth factor receptor (EGFR) inhibitor neratinib. The method is characterized in that 6-[(E)-4-(dimethylamino)-2-butenylamido]-7-ethoxy-4-chloro-3-quinolylmethylnitrile (I) continuously reacts with 2-chloro-3-aminophenol (II) and dichloromethylpyridine hydrochloride (III) to temporarily obtain an intermediate (E)-N-{4-[3-chloro-4-hydroxyanilino]3-cyno-5-ethoxy-6-quinolyl}-4-dimethylamino-2-butenylamide (IV) in order to finally obtain neratinib. The preparation method adopts a continuous reaction process, and dichloromethylpyridine hydrochloride (III) is a catalyst in the first stage of the continuous reaction and is a reaction reagent in the second stage of the continuous reaction, so the method has the advantages of simple and easily available raw materials, high yield, mild reaction conditions, extremely small amount of a generated waste liquid, and high facilitation of industrial production of the above raw medicine.
Owner:SHANGHAI STEPPHARM CO LTD
Method for synthetizing polyamic acid resin from 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene
The invention discloses a method for synthetizing polyamic acid resin from 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene. The method is characterized in that 1,3-bis(4-fluorobenzoyl) benzene and 3-aminophenol react to prepare the 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene; then, the 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene and pyromellitic dianhydride react to synthetize the polyamic acid resin; after extruding coating, imine formation is performed to prepare a polyimide thin film. The thickness of the prepared thin film is thin and can reach 11 to 13 mu m; the thin film is suitable for flexible circuit material preparation; the contraction rate is low; the volume resistivity reaches 10<12> Omega.m or higher; the surface resistivity reaches 10<14> Omega.
Owner:JIANGSU YABAO INSULATION MATERIAL
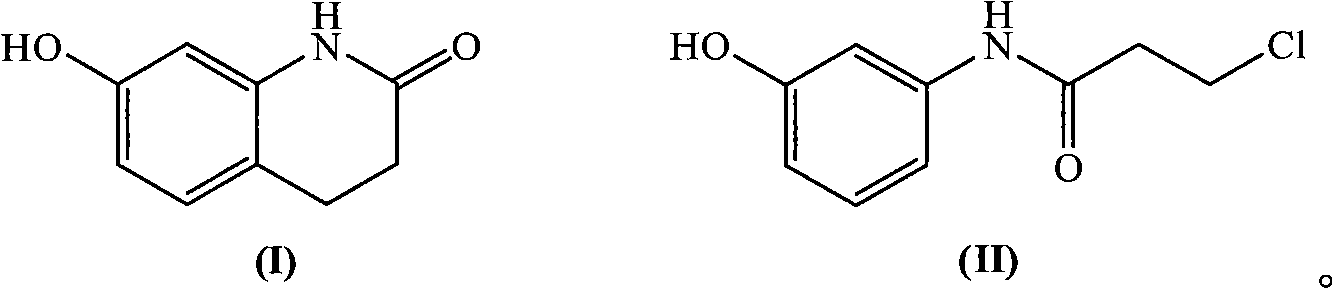
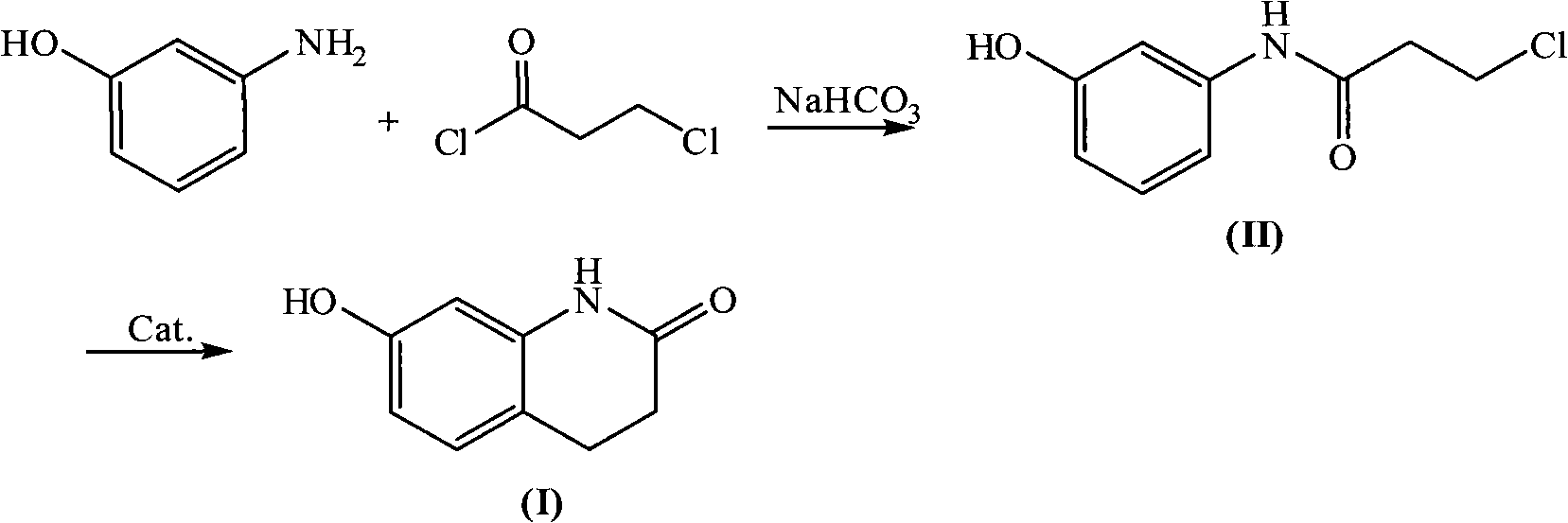
Novel synthetic method of 7-hydroxy-3,4-dihydroquinolines
ActiveCN101302195AEasy to separate and recycleGood choiceOrganic chemistryChemical recyclingAlkyl transferSynthesis methods
Owner:ZHEJIANG BENLI TECH CO LTD
Agent for oxidatively dyeing hair containing specific combinations of developers and couplers
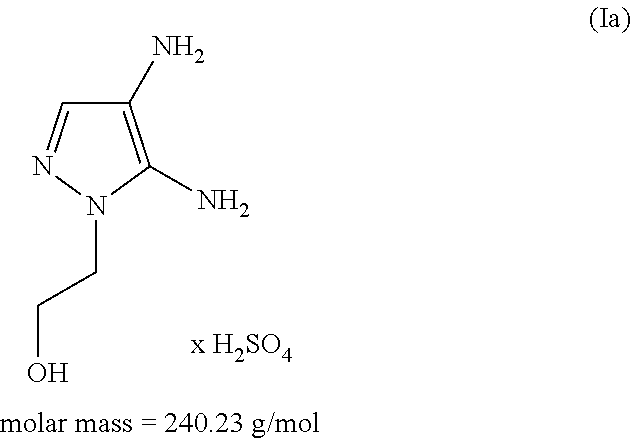
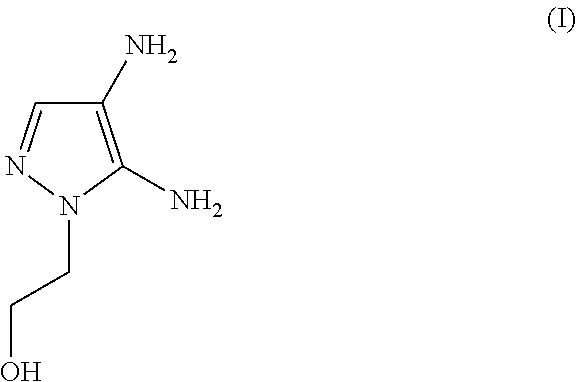
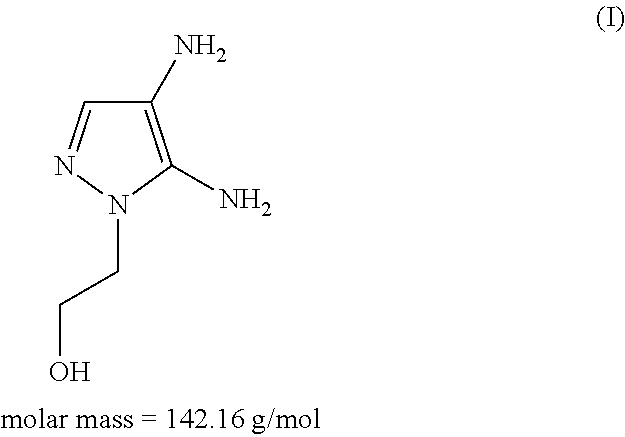
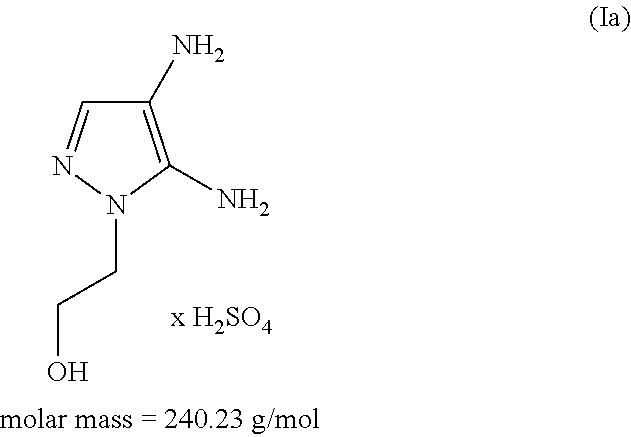
The subject matter of the present invention relates to an agent for oxidatively dyeing keratinous fibers, comprising, in a cosmetic carrier, (A) 4,5-diamino-1-(2-hydroxyethyl)-1H-pyrazole and / or one of the physiologically acceptable salts thereof as a developer, (B) at least one p-diaminobenzene derivative from the group of p-toluylenediamine, 2-(2,5-diaminophenyl)ethanol, and / or one of the physiologically acceptable salts thereof as a developer, (C) at least one m-diaminobenzene derivative from the group of 2-(2,4-diaminophenoxy)ethanol, 1-methoxy-2-amino-4-(2′-hydroxyethylamino)benzene, 2,6-bis(2′-hydroxyethylamino)-1-methylbenzene, and / or one of the physiologically acceptable salts thereof as a coupler, and (D) at least one m-aminophenol derivative from the group 3-aminophenol, 5-amino-2-methylphenol, and / or one of the physiologically acceptable salts thereof as a coupler, wherein the molar ratio of all developers of group (A) included in the agent to all developers of group (B) included in the agent, i.e., the molar ratio (A) / (B), has a value of at least 1.2.
Owner:HENKEL KGAA
A fluorescent probe for detecting hydrogen peroxide and its preparation method and application
ActiveCN105647518BGood choiceImprove universalityMethine/polymethine dyesOrganic chemistryFluorescenceSynthesis methods
Owner:SHANDONG NORMAL UNIV
Method for preparing paint used for envelope material
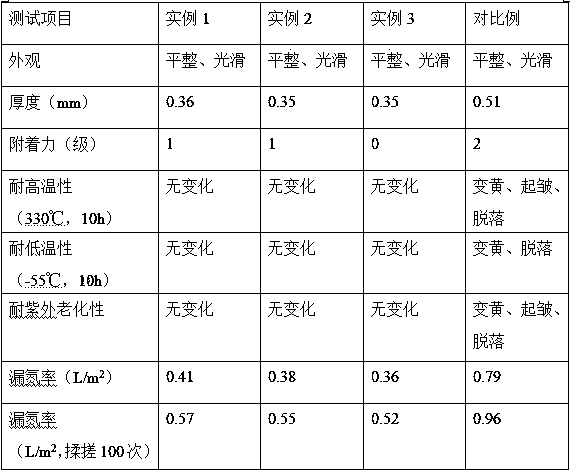
InactiveCN109181438AHigh curing crosslinking activityGood compatibilityPolyamide coatingsEpoxy resin coatingsDispersion stabilityUltraviolet lights
The invention relates to the technical field of paint preparation, and concretely relates to a method for preparing a paint used for an envelope material. The method uses 3-aminophenol and ethyl acetoacetate as raw materials for a reaction to obtain coumarin crystal, and the coumarin crystal is continuously reacted with 2,3-epoxypropyltrimethyl ammonium chloride and acryloyl chloride to obtain ananti-ultraviolet light stabilizer, diethylenetriamine, diethanolamine and toluene sulphonatic acid are mixed to obtain a silicon resin modified PAE emulsion, and the silicon resin modified PAE emulsion, the anti-ultraviolet light stabilizer, bisphenol A epoxy resin and the like are reacted to obtain the paint used for the envelope material. The prepared coumarin can promote the curing cross-linking, and improves the ultraviolet aging resistance of the paint, the silicon resin has high-low temperature resistance and good weather resistance, the interfacial compatibility of the paint and the envelope material can be improved, the PAE emulsion penetrates into the gap of the aramid fiber in the envelope material, so that the dispersion stability of the paint for the envelope material during curing is improved, and the application prospect is wide.
Owner:FOSHAN SENANG BIO TECH CO LTD
Preparation method of environment-friendly photoresist
InactiveCN109358473APromote degradationHigh light transmittancePhotosensitive materials for photomechanical apparatusResistPhosphoric acid
The invention provides a preparation method of an environment-friendly photoresist. The method comprises the following steps that 3-aminophenol and phosphoric acid are stirred and mixed uniformly, ethyl acetoacetate is slowly added dropwise, temperature-rising stirring and condensation backflow are conduced, and a reaction is conducted; a mixture is filtered in a funnel through filtering paper andwashed; drying is conducted to obtain a product A; the product A and tetrahydrofuran are mixed and heated, and condensation backflow and stirring are conducted; cooling is conducted, acryloyl chloride is added dropwise, nitrogen is introduced after dropwise addition is completed, and a stirring reaction is conducted; deionized water is added, and white solids are precipitated and washed; drying is conducted to obtain a product B; the product B, hyperforin perforatum alkali, acrylic anhydride, N-acetylcysteine, casein, dithiothreitol, benzil dimethyl ether and ethanol are mixed and uniformly stirred in a dark environment, filter residues are removed through filtering, and the environment-friendly photoresist is obtained. The environment-friendly photoresist prepared by means of the methodis high in light transmittance, good in light transmittance performance, beneficial to application, high in graphic contrast ratio and high in resolution, and has a great photoetching effect.
Owner:嘉兴市海德姆智能电气有限公司
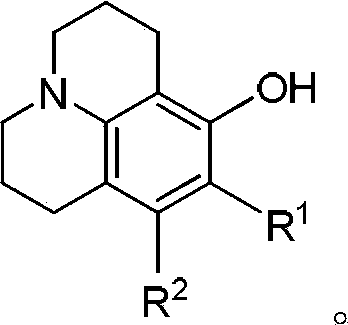
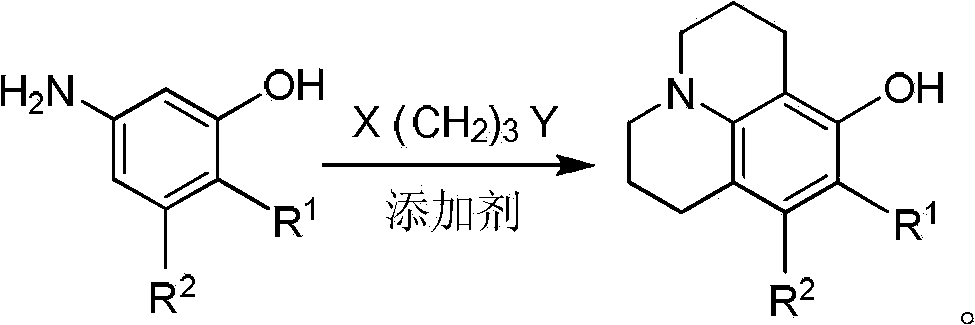
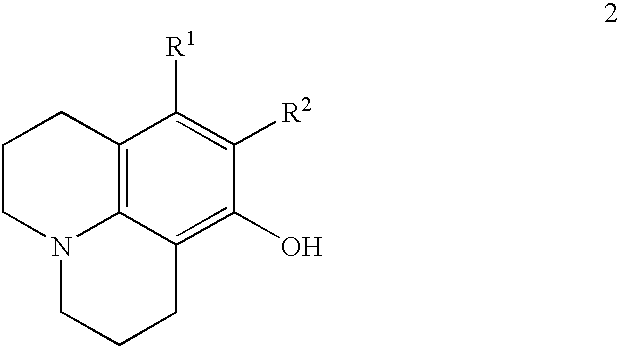
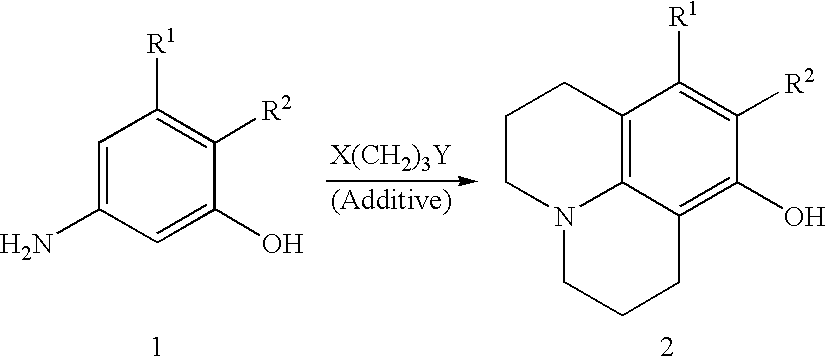
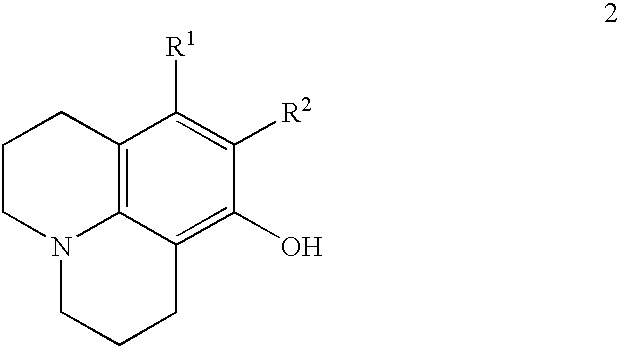
Method for preparing 8-hydroxy julolidine and derivative thereof
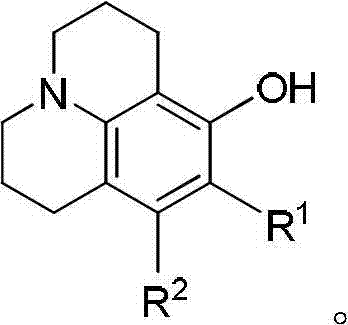
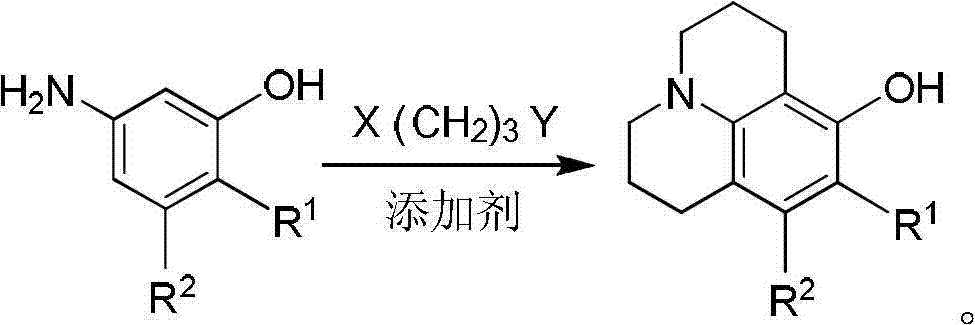
The invention discloses a method for preparing 8-hydroxy julolidine and a derivative thereof. The method comprises the following steps of: (1) solving 3-aminophenol compound and X(CH2)3Y in an organic solvent S1, adding alkali B1, performing intramolecular reaction, reacting until the 3-aminophenol compound fully converts into an intermediate, and processing after finishing the reaction to obtain an intermediate; and (2) adding the intermediate obtained in the step (1) and alkali B2 into an organic solvent S2, performing intramolecular reaction, and processing until finishing the reaction to obtain the objective product. The method for preparing the 8-hydroxy julolidine and the derivative thereof is simple in steps; by strictly controlling the reaction stages, occurrence of disubstituted side reaction and oxidation side reaction is reduced, and productivity ratio of the objective product is improved; and high-poisonous and high-polluted substrate or reagent is avoided in the whole reaction process, which is suitable for industrialization.
Owner:ZHEJIANG UNIV
Cobalt-containing monatomic catalyst as well as macro preparation method and application thereof
PendingCN114425391AIncrease the amount of feedCost-effectivePhysical/chemical process catalystsElectrodesFiberElectronic structure
The invention discloses a cobalt-containing monatomic catalyst as well as a macro preparation method and application thereof. The preparation method comprises the following specific steps: 1) dispersing 3-aminophenol and hexamethylenetetramine in water, and reacting at 75-95 DEG C for 24 hours by taking hexadecyl trimethyl ammonium bromide as a morphology guiding agent, so as to obtain phenolic resin fibers; and 2) stirring the obtained phenolic resin fiber and a cobalt salt solution at normal temperature for 12 hours at the stirring speed of 200rpm, centrifuging, taking the precipitate, freeze-drying, carbonizing the freeze-dried product to 800-950 DEG C in an inert atmosphere, and keeping the temperature for 60 minutes to obtain the cobalt-containing monatomic catalyst. Compared with the prior art, the method disclosed by the invention is simple to operate and easy for large-scale production, and the prepared carbon nanofiber loaded cobalt monatomic material has the advantages of maximized atom utilization rate, unique electronic structure, good conductivity, high catalytic activity and the like.
Owner:NANJING NORMAL UNIVERSITY
Method for preparing 8-hydroxy julolidine and derivative thereof
The invention discloses a method for preparing 8-hydroxy julolidine and a derivative thereof. The method comprises the following steps of: (1) solving 3-aminophenol compound and X(CH2)3Y in an organic solvent S1, adding alkali B1, performing intramolecular reaction, reacting until the 3-aminophenol compound fully converts into an intermediate, and processing after finishing the reaction to obtain an intermediate; and (2) adding the intermediate obtained in the step (1) and alkali B2 into an organic solvent S2, performing intramolecular reaction, and processing until finishing the reaction to obtain the objective product. The method for preparing the 8-hydroxy julolidine and the derivative thereof is simple in steps; by strictly controlling the reaction stages, occurrence of disubstituted side reaction and oxidation side reaction is reduced, and productivity ratio of the objective product is improved; and high-poisonous and high-polluted substrate or reagent is avoided in the whole reaction process, which is suitable for industrialization.
Owner:ZHEJIANG UNIV
Agent for the oxidative dyeing of hair, containing specific combinations of developers and couplers
An agent for oxidatively dyeing keratinous fibers includes in a cosmetic carrier: (A) 4,5-diamino-1-(2-hydroxyethyl)-1H-pyrazole and / or one of the physiologically acceptable salts thereof as developer; (B) at least one p-aminophenol derivative from the group comprising p-aminophenol, 4-amino-3-methylphenol, and / or the physiologically acceptable salts thereof as developer; (C) at least one m-diaminobenzene derivative from the group comprising 2-(2,4-diaminophenoxy)ethanol, 1-methoxy-2-amino-4-(2′-hydroxyethylamino)benzene, 2,6-bis(2′-hydroxyethylamino)-1-methylbenzene, and / or one of the physiologically compatible salts thereof as coupler; and (D) at least one m-aminophenol derivative from the group 3-aminophenol, 5-amino-2-methylphenol and / or one of the physiologically acceptable salts thereof as coupler, wherein the molar ratio of all the developers of group (A) included in the agent to all the developers of group (B) included in the agent, i.e., the molar ratio (A) / (B), has a value of at least 1.2.
Owner:HENKEL KGAA
Method for detecting bacterial AmpC enzyme using paper disk containing 3-amino phenol borate
InactiveCN100432234CAvoid missing detectionIncrease positive rateMicrobiological testing/measurementBoric acid3-Aminophenol
This invention discloses a method for testing bacteria AmpC enzyme with paper containing 3-amino-phenol-boric acid. It prepares APB solution first and adds APB on the CAZ, CTX and FOX paper without APB to apply APB paper reinforcement method to carry out paper method drug hypersensitive test to test the AmpC enzyme generated by bacteria. APB can enlarge the microbiostatic ring of CAZ, CTX or FOX paper to the generated AmpC enzyme bacteria so as to judge the existence of AmpC enzyme by testing the change of the diameter of the ring to avoid missed test brought with using the single base material to increase the positive rate.
A single high-yield method for preparing monodisperse nitrogen-doped ordered mesoporous carbon spheres with a particle size of 100-800 nm
ActiveCN105565296BIncrease concentrationAchieve outputMaterial nanotechnologyCarbon preparation/purificationAlcoholSorbent
The invention discloses a method for preparing mono-dispersed N-doped ordered mesoporous carbon spheres with the particle size of 100-800 nm at high yield in a single-pass mode. According to the method, F127, 3-aminophenol and NH3.H2O serve as a template, a carbon and nitrogen source and a catalyst respectively, and the mono-dispersed N-doped ordered mesoporous carbon spheres with the particle size of 100-800 nm are produced at high yield in the single-pass mode on a large scale by regulating and controlling product morphology through ethyl alcohol. The preparing method is easy to implement, the concentration of the carbon and nitrogen source in the system is high, dosage of solvent is greatly reduced, several grams of N-doped ordered mesoporous carbon spheres can be obtained at high yield in the single-pass mode, the obtained N-doped ordered mesoporous carbon spheres are small in size distribution range, good in monodispersity and regular in pore structure and can be directly used as a catalyst, adsorbent and the like.
Owner:SHAANXI NORMAL UNIV +1
A novel preparation method of epidermal growth factor receptor (EGFR) inhibitor neratinib
ActiveCN105461689BOrganic active ingredientsOrganic chemistryHuman epidermal growth factor receptor3-Aminophenol
The invention relates to a preparation method of an epidermal growth factor receptor (EGFR) inhibitor neratinib. The method is characterized in that 6-[(E)-4-(dimethylamino)-2-butenylamido]-7-ethoxy-4-chloro-3-quinolylmethylnitrile (I) continuously reacts with 2-chloro-3-aminophenol (II) and dichloromethylpyridine hydrochloride (III) to temporarily obtain an intermediate (E)-N-{4-[3-chloro-4-hydroxyanilino]3-cyno-5-ethoxy-6-quinolyl}-4-dimethylamino-2-butenylamide (IV) in order to finally obtain neratinib. The preparation method adopts a continuous reaction process, and dichloromethylpyridine hydrochloride (III) is a catalyst in the first stage of the continuous reaction and is a reaction reagent in the second stage of the continuous reaction, so the method has the advantages of simple and easily available raw materials, high yield, mild reaction conditions, extremely small amount of a generated waste liquid, and high facilitation of industrial production of the above raw medicine.
Owner:SHANGHAI STEPPHARM CO LTD
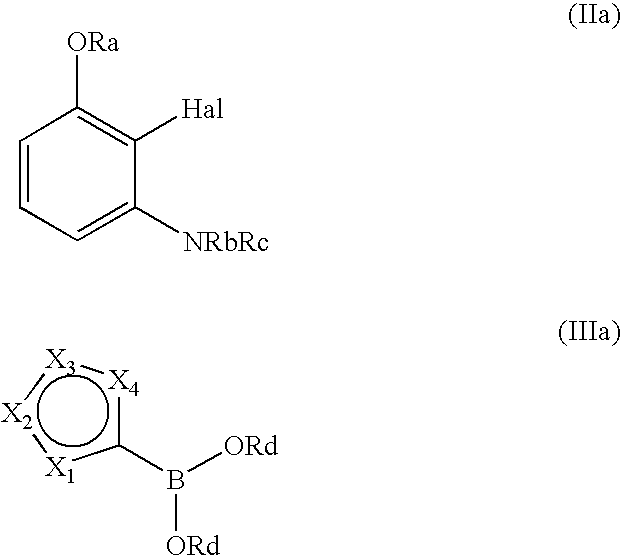
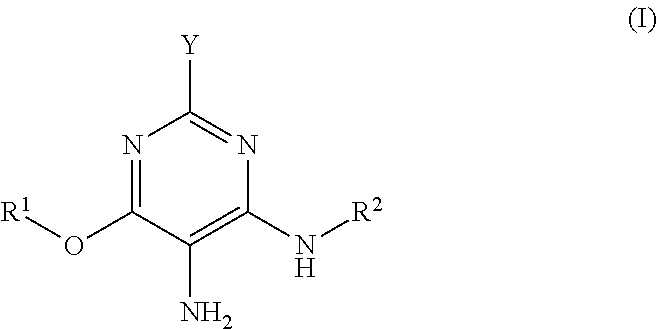
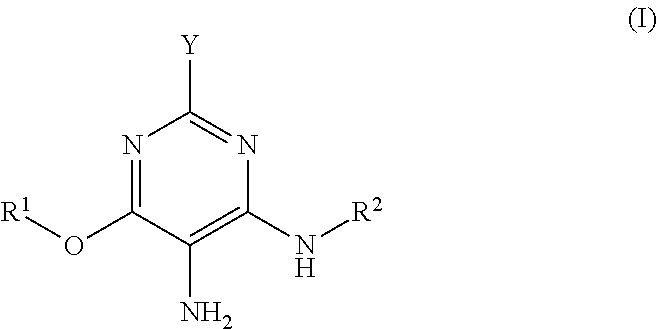
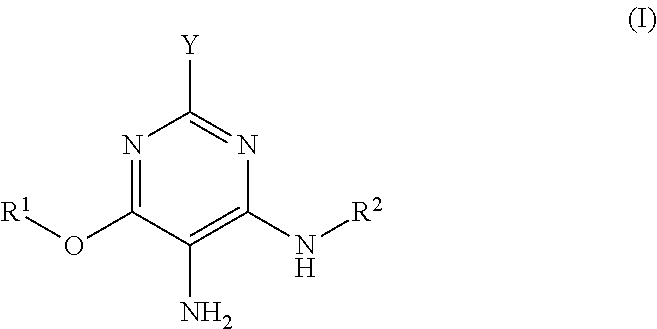
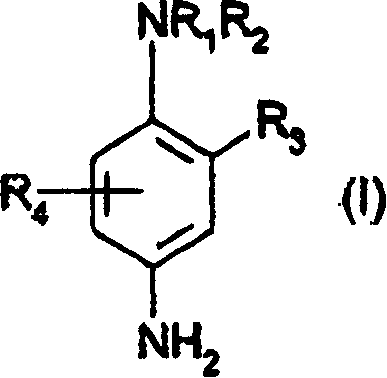
3-aminophenol derivatives and dyes that contain these compounds while serving to dye keratin fibers
InactiveUS7132001B2Readily water-solubleHigh color intensityCosmetic preparationsHair cosmeticsFiberWater soluble
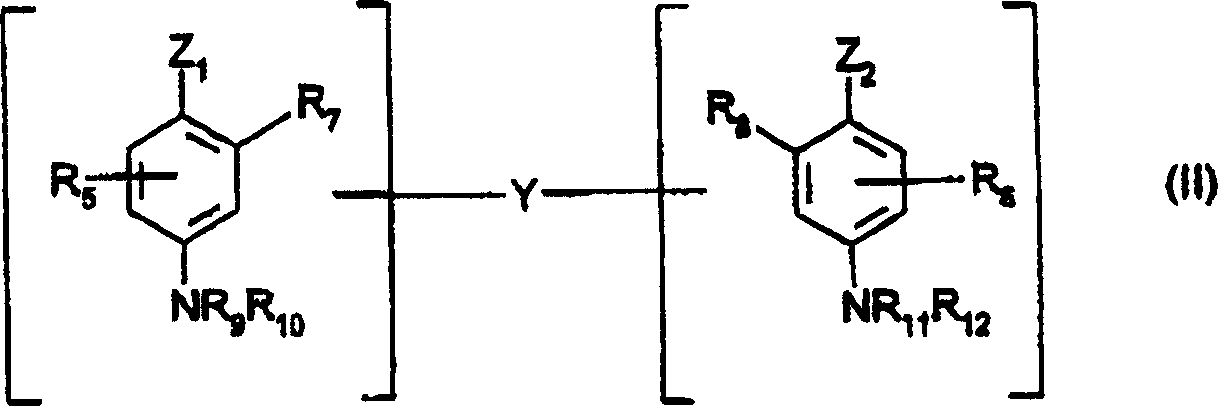
The object of the present patent application are 3-aminophenol derivatives of formula (I) or the physiologically compatible, water-soluble salts thereofand colorants based on a developer-coupler combination containing these compounds.
Owner:WELLA AG
Phosphine-containing aromatic diamine compound, preparation method and application thereof
ActiveCN101531678BImprove heat resistanceHigh glass transition temperatureGroup 5/15 element organic compoundsPhosphine oxideGrignard reaction
The invention discloses an aromatic diamine compound containing aryl phosphine groups, a prepration method and an application thereof. The structural general formula of the compound is shown in a formula I. The compound takes 3,5-difluoro-bromobenzene and diphenylphosphinic chloride as raw materials, firstly prepares 3,5-difluorophenyl diphenyl phosphine oxide by Grignard reaction and then continuously reacts with 3-aminophenol for preparation. The compound has smaller steric effect and has great value for improving the heat resistance performance of atomic oxygen resistant polyimide thin film materials and improving the visible light transparency. In addition, polyimide phosphine-containing polymer materials prepared by utilizing the phosphine-containing aromatic diamine compound have good atomic oxygen resistance performance, higher glass transition temperature and better visible light transparency, thereby being capable of being applied in the manufacturing of satellites and other aircraft parts in the LEO environment.
Owner:INST OF CHEM CHINESE ACAD OF SCI
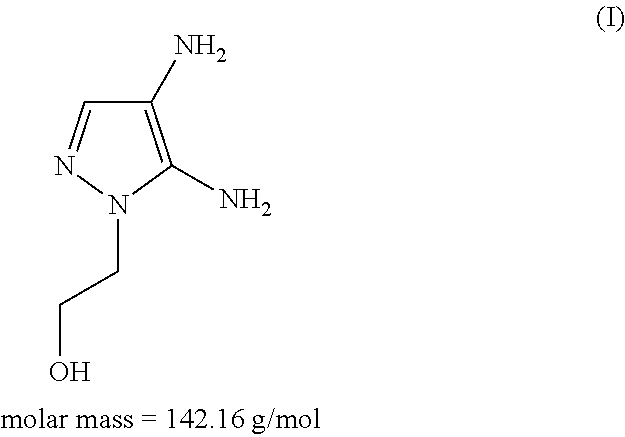
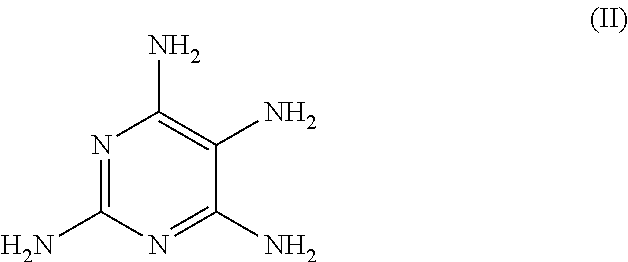
Hair dye agent comprising aminopyrimidine derivatives
The application relates to agents for dyeing keratin fibers. The agent can be present in a cosmetic carrier and includes at least one compound of formula (I) and / or a physiologically compatible salt of this compound,in which R1 is a C1-C4 alkyl group, R2 is a hydrogen atom, a C1-C8 alkyl group, a C2-C6 alkenyl group, a C1-C6 hydroxyalkyl group, a C2-C6 polyhydroxyalkyl group, a C1-C6 alkoxy group, a C1-C6 alkylamino group, a di-C1-C6 alkylamino group, a C1-C6 polyalkoxy group, a C1-C6 alkylamido-(C1-C6) alkyl group, wherein the amide function can be substituted by one or two groups X, or is an aryl group, Y is an NH2 or an OH group, at least one coupler component selected from 3-amino-2-methylamino-6-methoxypyridine, 3-amino-6-methylphenol, 3-amino-2-hydroxypyridine, 1,3-bis-(2,4-diaminophenoxy)propane, 2,7-dihydroxynaphthalene, 2-methylresorcinol, 2,5-dimethylresorcinol, 4-chlororesorcinol, 3-aminophenol, 2-amino-3-hydroxypyridine, 2-chloro-6-methyl-3-aminophenol, 2,6-dihydroxy-3,4-dimethylpyridine, 2-({3-[(2-hydroxyethyl)amino]-4-methoxy-5-methylphenyl}amino)ethanol, 2-({3-[(2-hydroxyethyl)amino]-2-methoxy-5-methylphenyl}amino)ethanol, 1-methoxy-2-amino-4-beta-hydroxy-ethylaminobenzene (Lehmann's blue), 2,4-diaminophenoxyethanol, 5-amino-4-chloro-o-cresol, 2,4-dichloro-m-aminophenol, 2,6-dihydroxy-3,4-dimethylpyridine and / or a physiologically compatible salt of these compounds.
Owner:HENKEL KGAA
Process for producing 1,3-bis(3-aminophenoxy) benzene
InactiveCN1457336AOrganic compound preparationAmino-hyroxy compound preparation3-AminophenolFluorobenzene
A process for industrially producing in a high yield 1,3-bis(3-aminophenoxy)benzene, which is effective as, e.g., a material for polyimides having high heat resistance, which comprises reacting 1,3-difluorobenzene with an alkali metal salt of 3-aminophenol.
Owner:三井化学FINE株式会社
Agent for the oxidative dyeing of hair, containing specific combinations of developers and couplers
An agent for oxidatively dyeing keratinous fibers includes in a cosmetic carrier: (A) 4,5-diamino-1-(2-hydroxyethyl)-1H-pyrazole and / or one of the physiologically acceptable salts thereof as developer; (B) at least one p-aminophenol derivative from the group comprising p-aminophenol, 4-amino-3-methylphenol, and / or the physiologically acceptable salts thereof as developer; (C) at least one m-diaminobenzene derivative from the group comprising 2-(2,4-diaminophenoxy)ethanol, 1-methoxy-2-amino-4-(2′-hydroxyethylamino)benzene, 2,6-bis(2′-hydroxyethylamino)-1-methylbenzene, and / or one of the physiologically compatible salts thereof as coupler; and (D) at least one m-aminophenol derivative from the group 3-aminophenol, 5-amino-2-methylphenol and / or one of the physiologically acceptable salts thereof as coupler, wherein the molar ratio of all the developers of group (A) included in the agent to all the developers of group (B) included in the agent, i.e., the molar ratio (A) / (B), has a value of at least 1.2.
Owner:HENKEL KGAA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Method for synthetizing polyamic acid resin from 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene Method for synthetizing polyamic acid resin from 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene](https://images-eureka.patsnap.com/patent_img/94a0a1cd-025e-4585-bd41-a458b0b5979b/BDA0001848421600000081.png)
![Method for synthetizing polyamic acid resin from 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene Method for synthetizing polyamic acid resin from 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene](https://images-eureka.patsnap.com/patent_img/94a0a1cd-025e-4585-bd41-a458b0b5979b/BDA0001848421600000082.png)
![Method for synthetizing polyamic acid resin from 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene Method for synthetizing polyamic acid resin from 1,3-bis[4-(3-aminophenoxy)benzoyl] benzene](https://images-eureka.patsnap.com/patent_img/94a0a1cd-025e-4585-bd41-a458b0b5979b/BDA0001848421600000091.png)