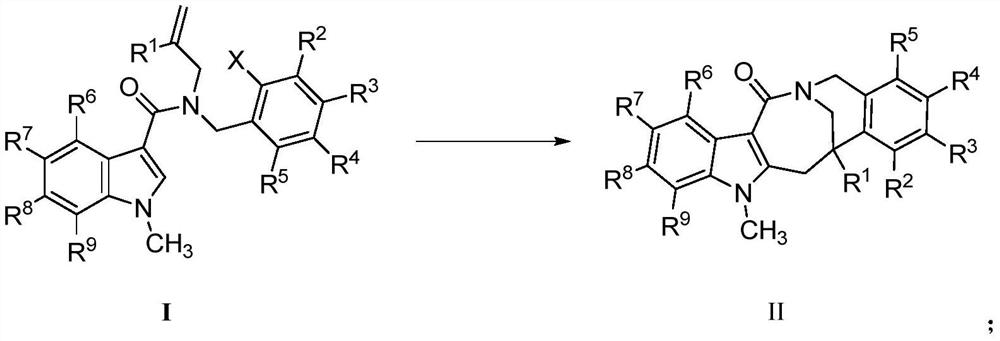
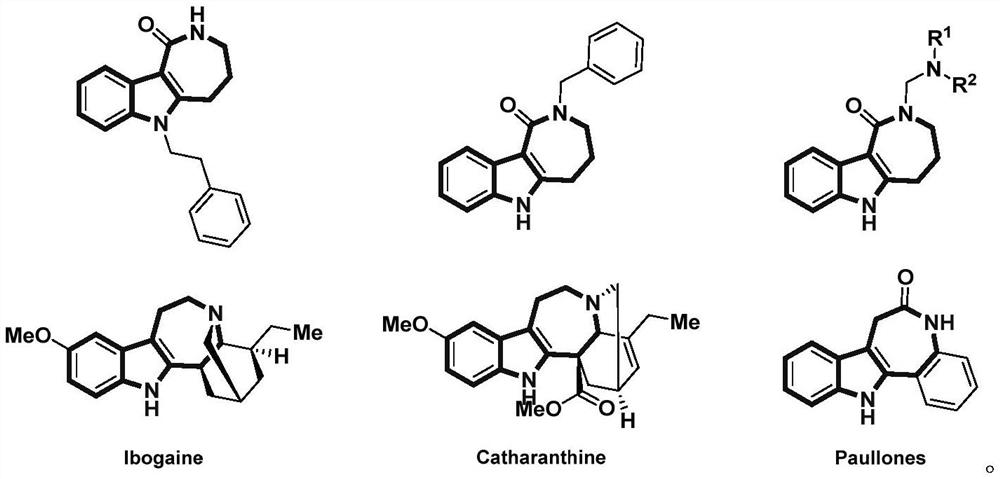
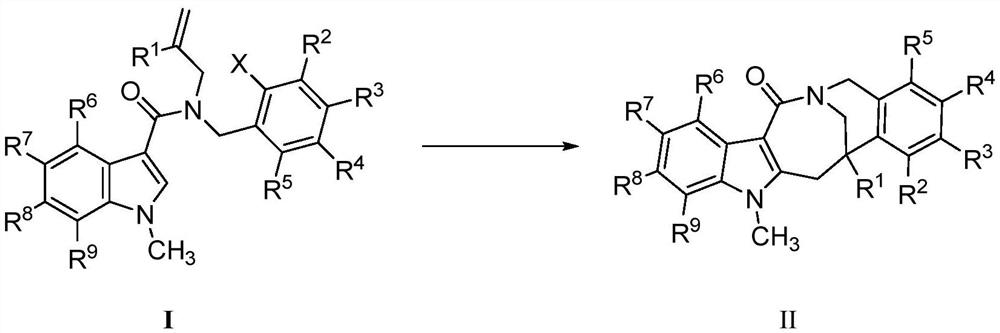
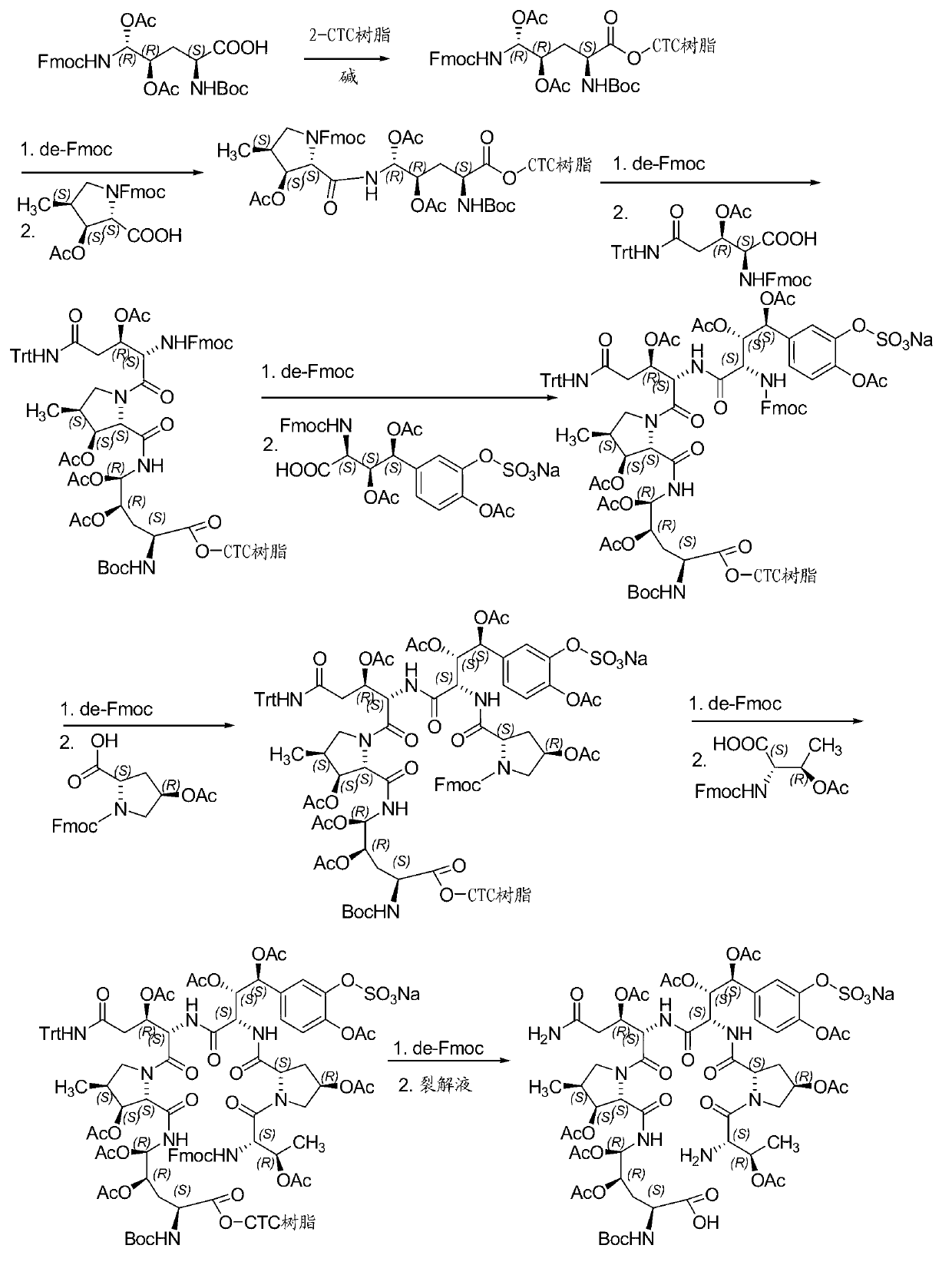
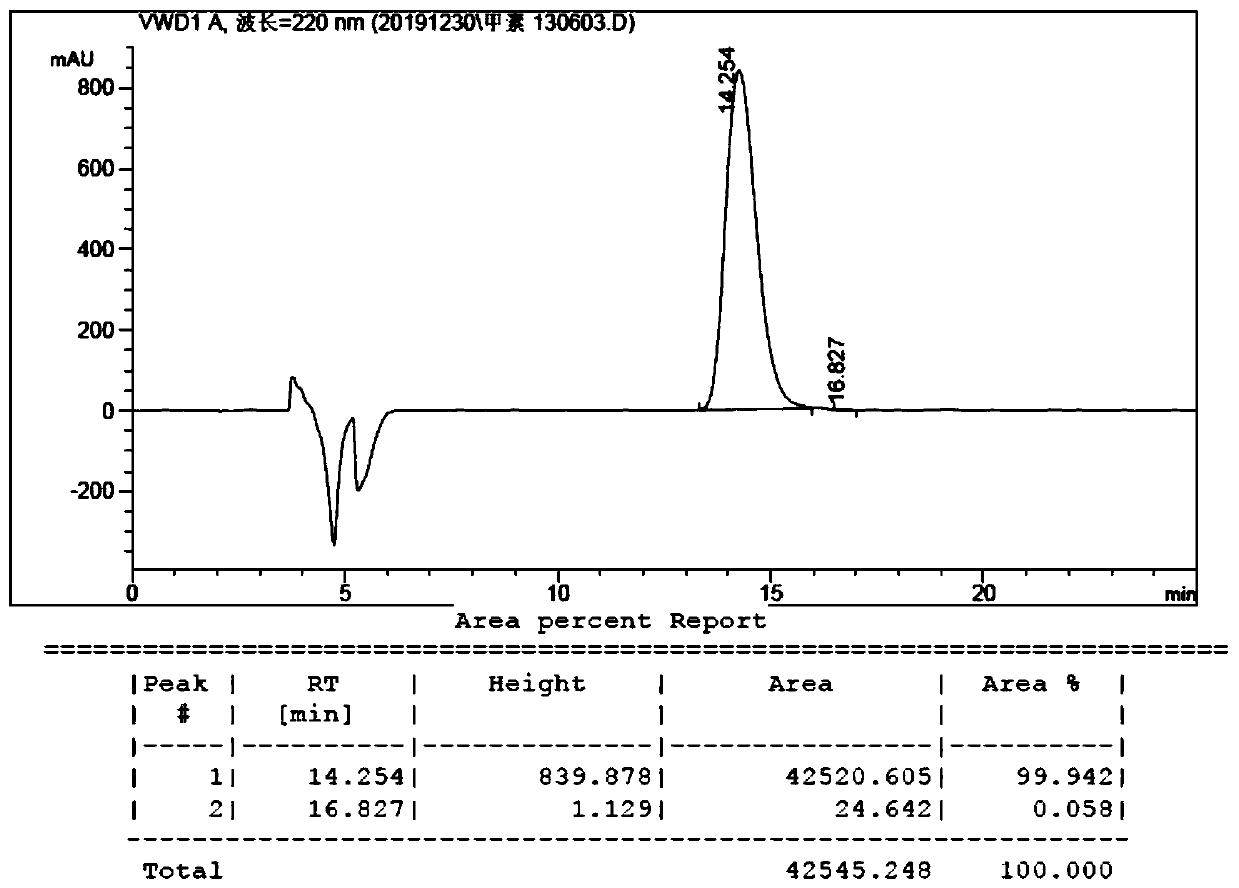
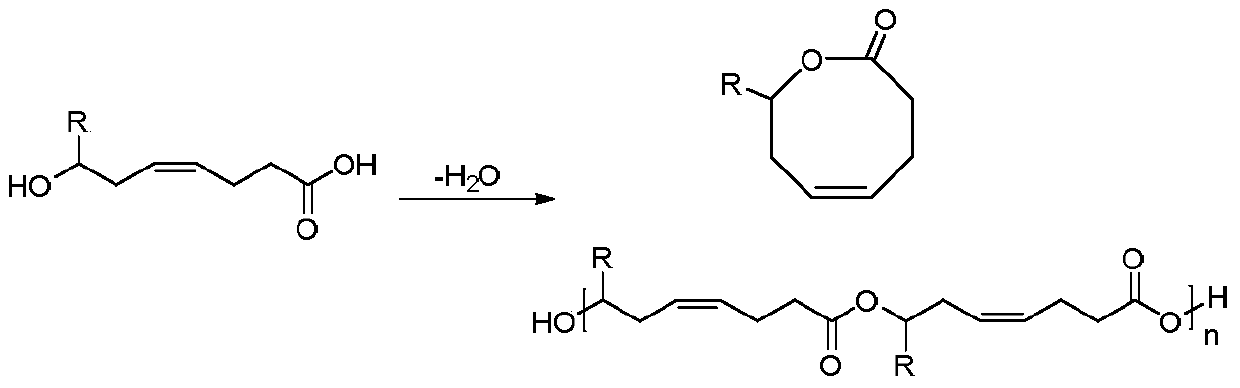
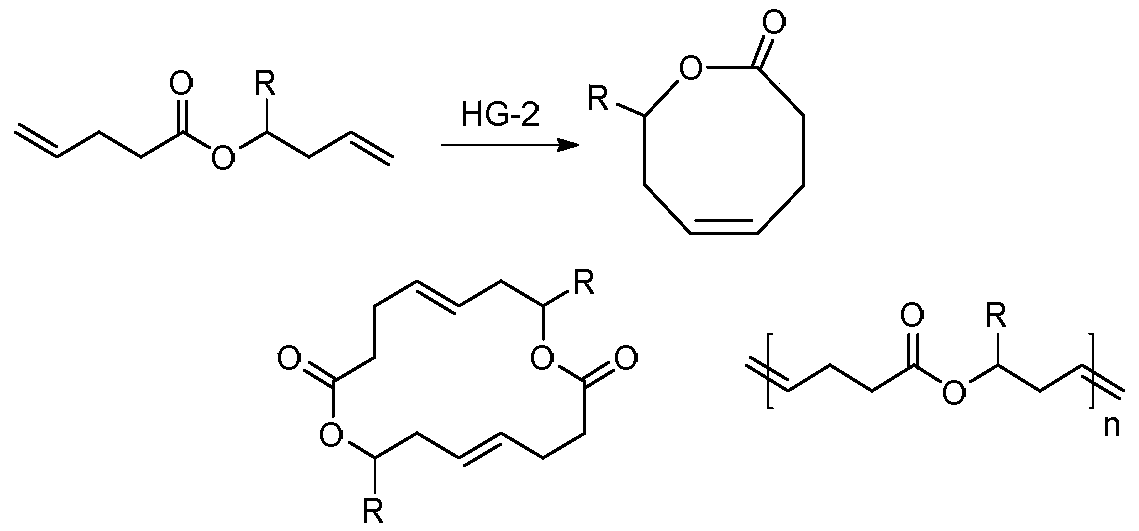
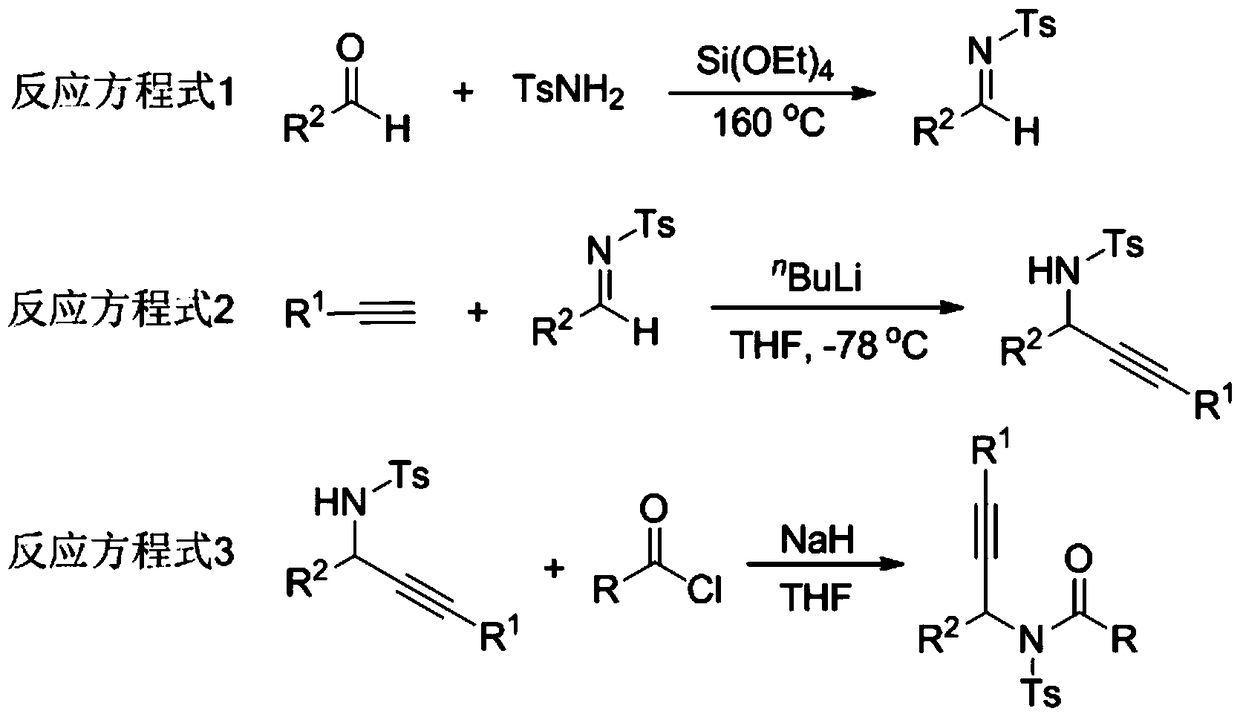
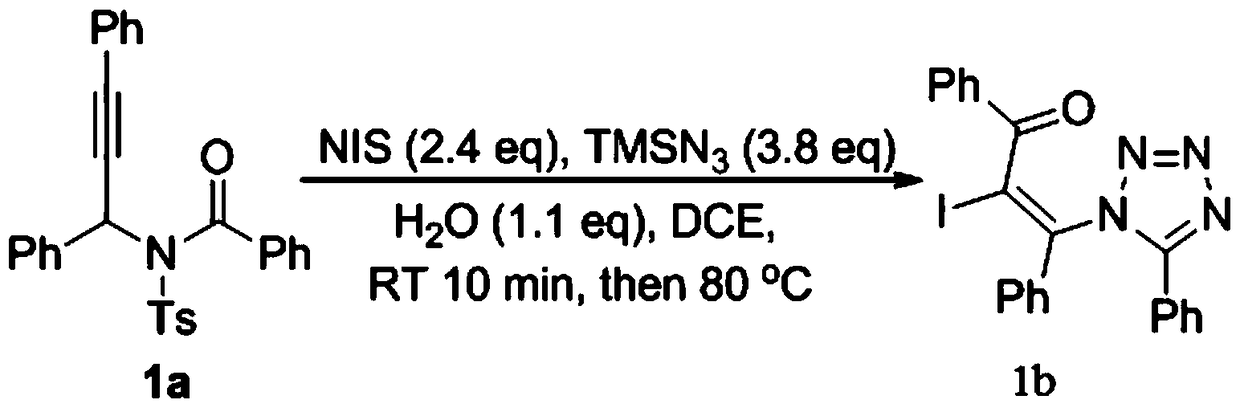
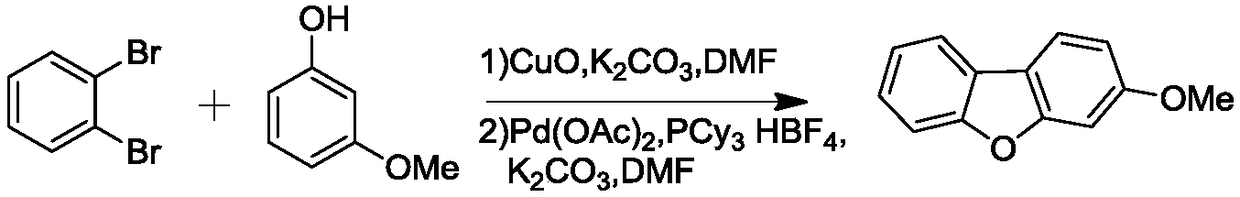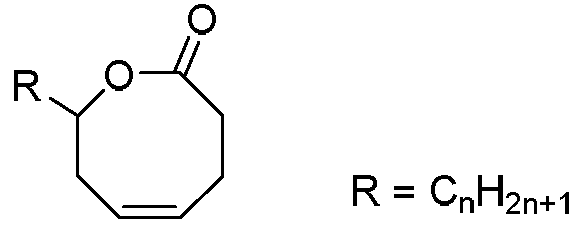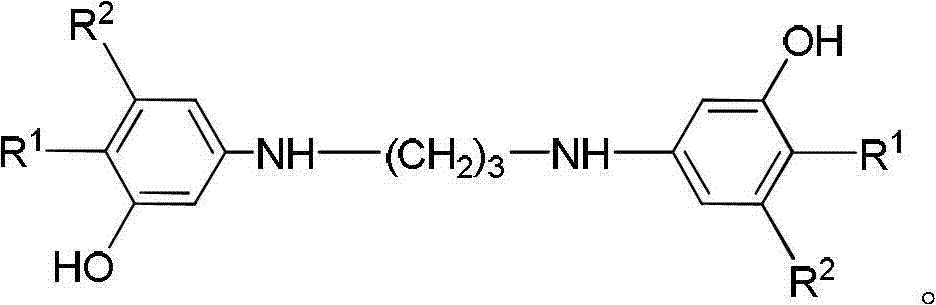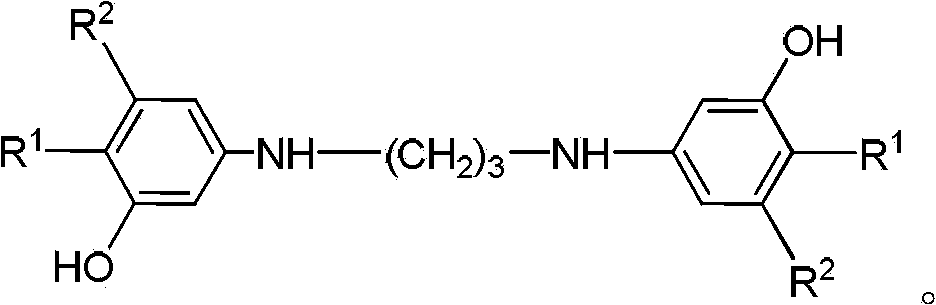Patents
Literature
35 results about "Intramolecular reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.
Palladium-catalyzed carbon-carbon bond forming reactions
ActiveUS7851658B2Organic compound preparationCarboxylic acid amides preparationCarbon–carbon bondAlkyne
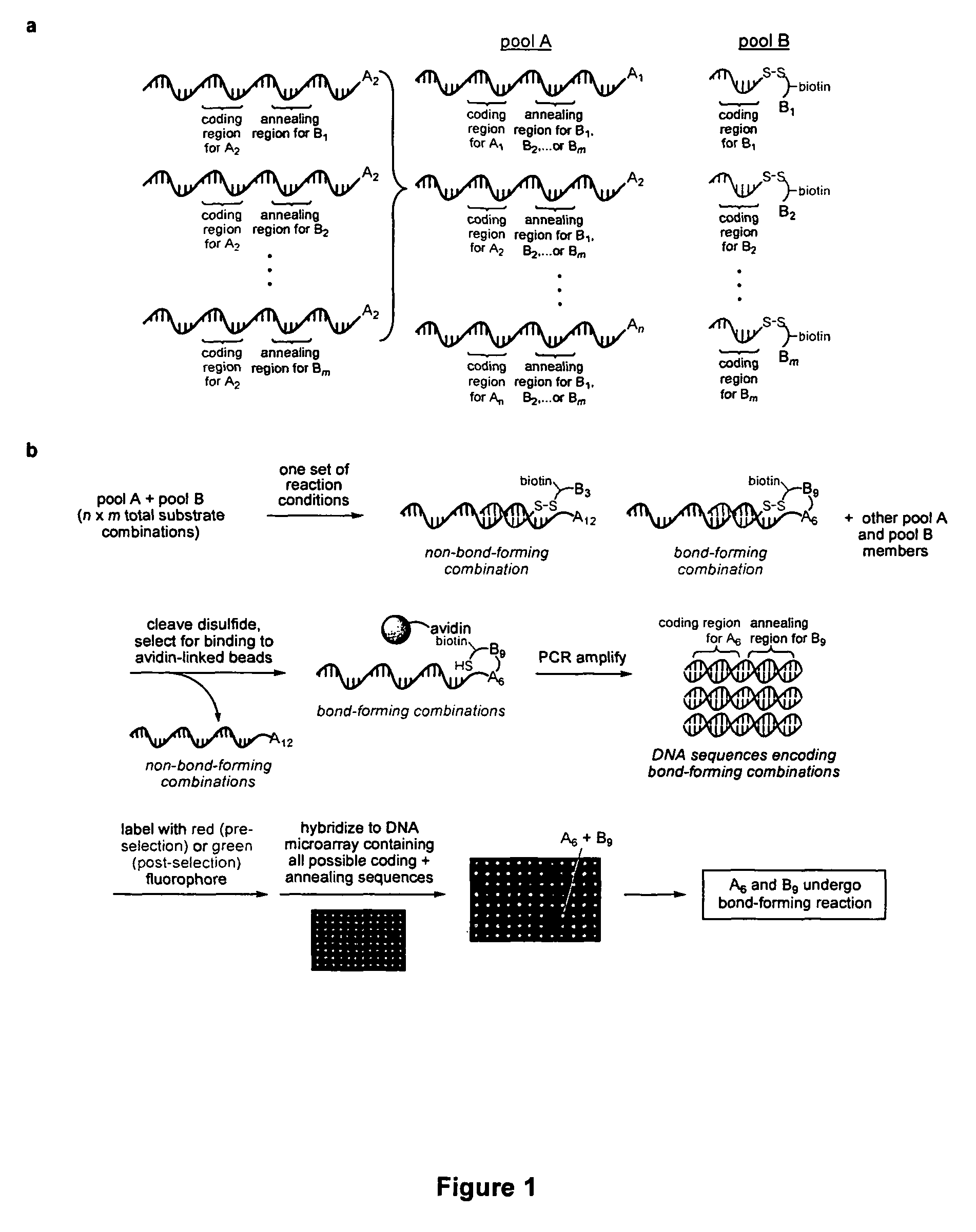
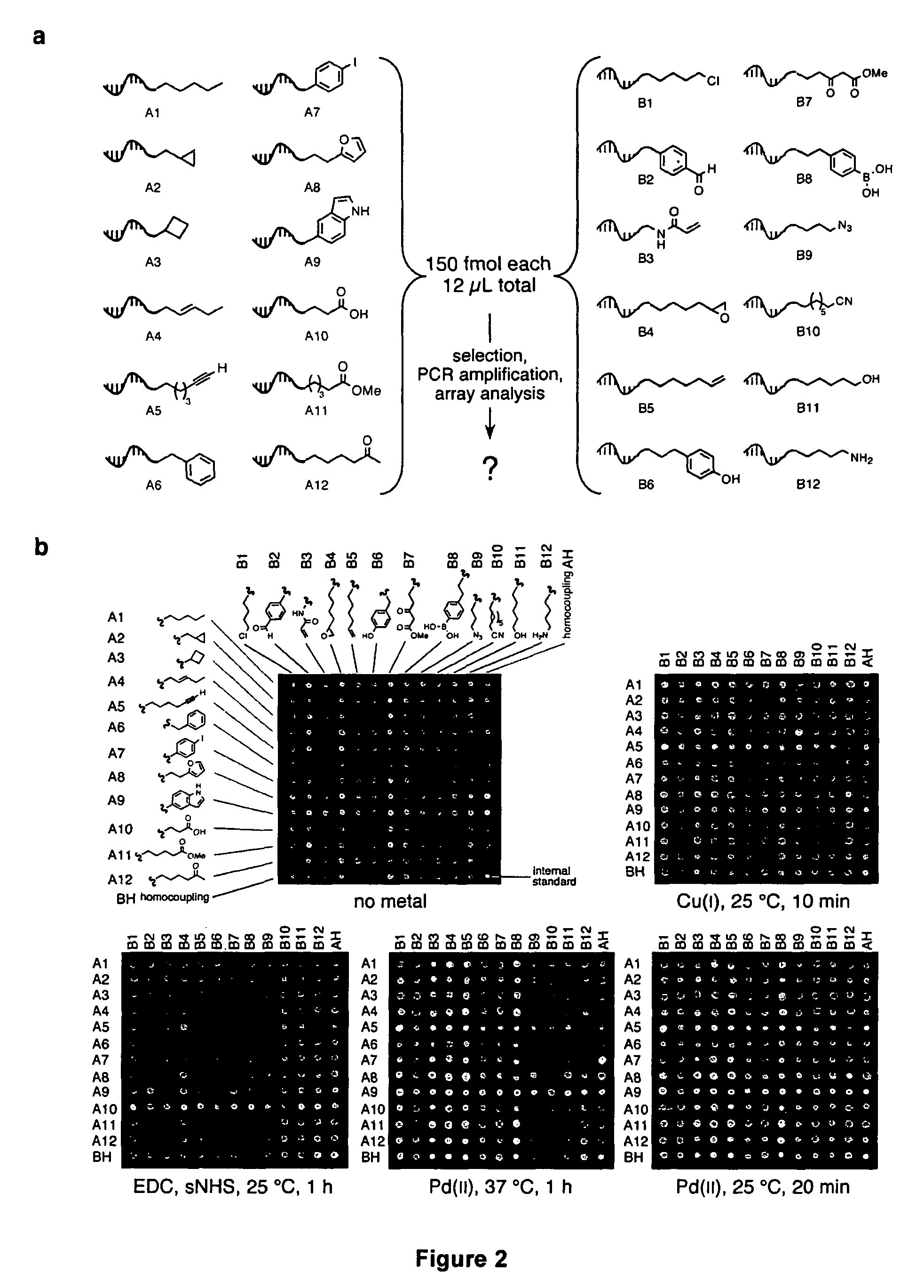
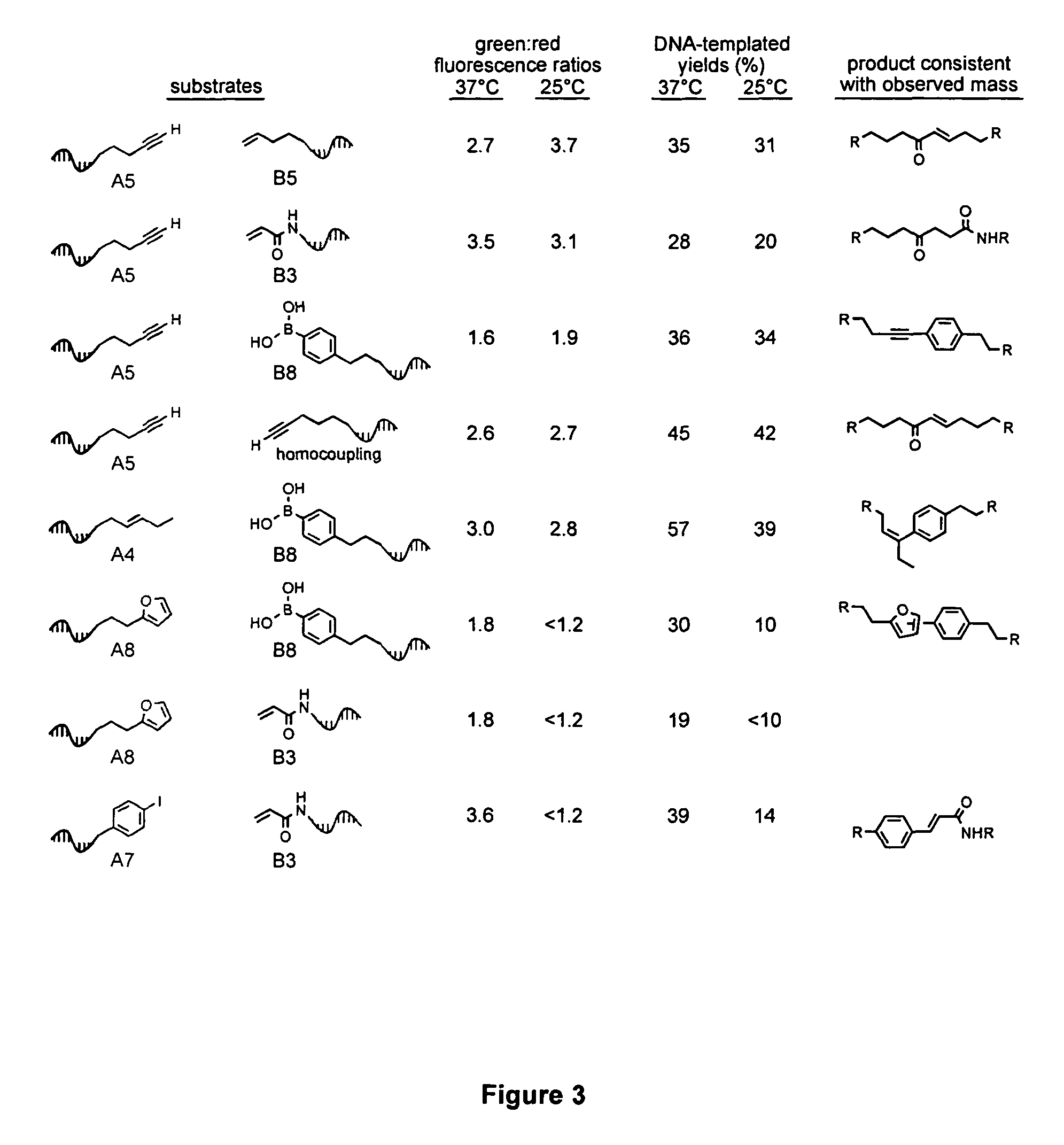
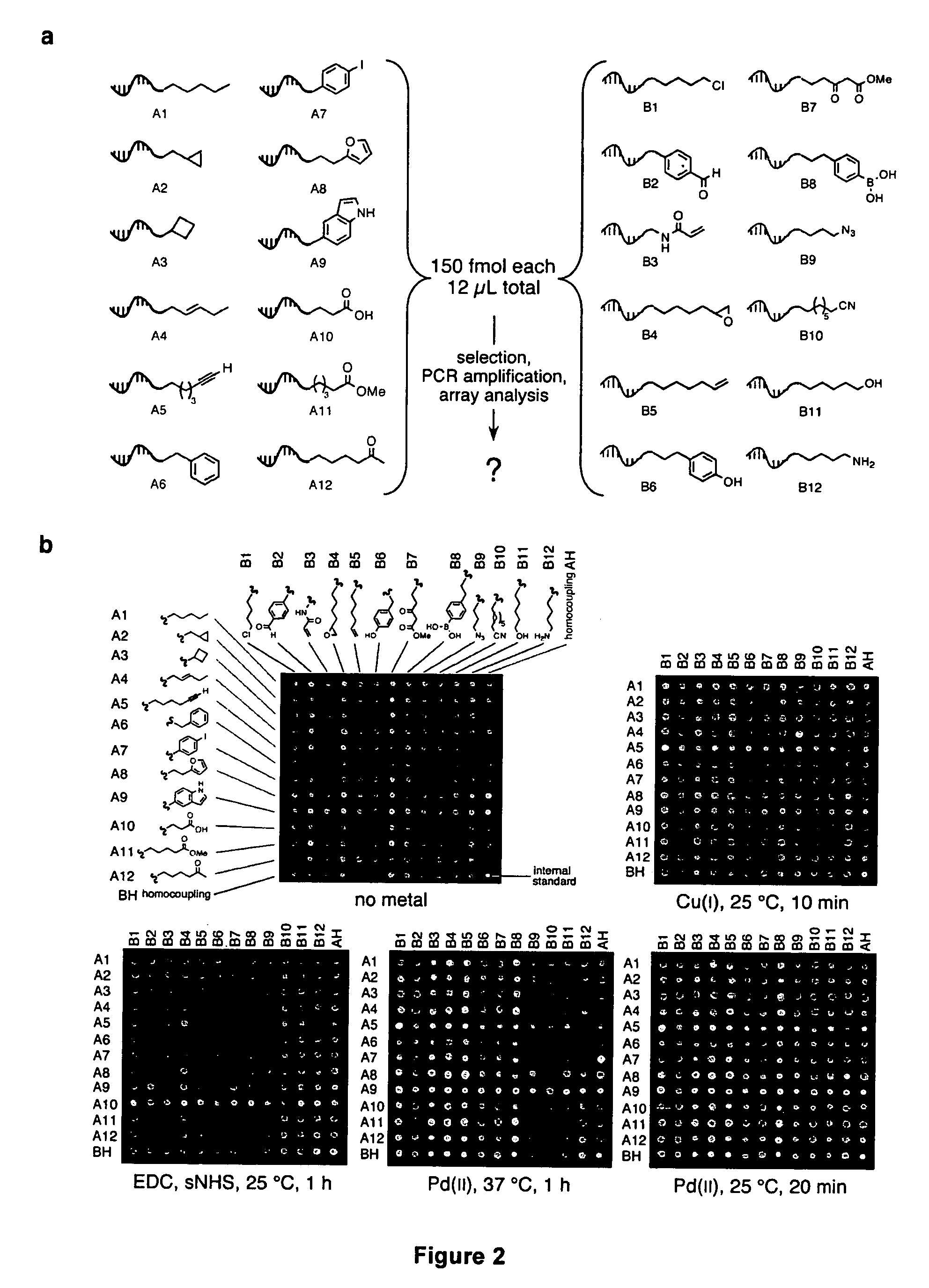
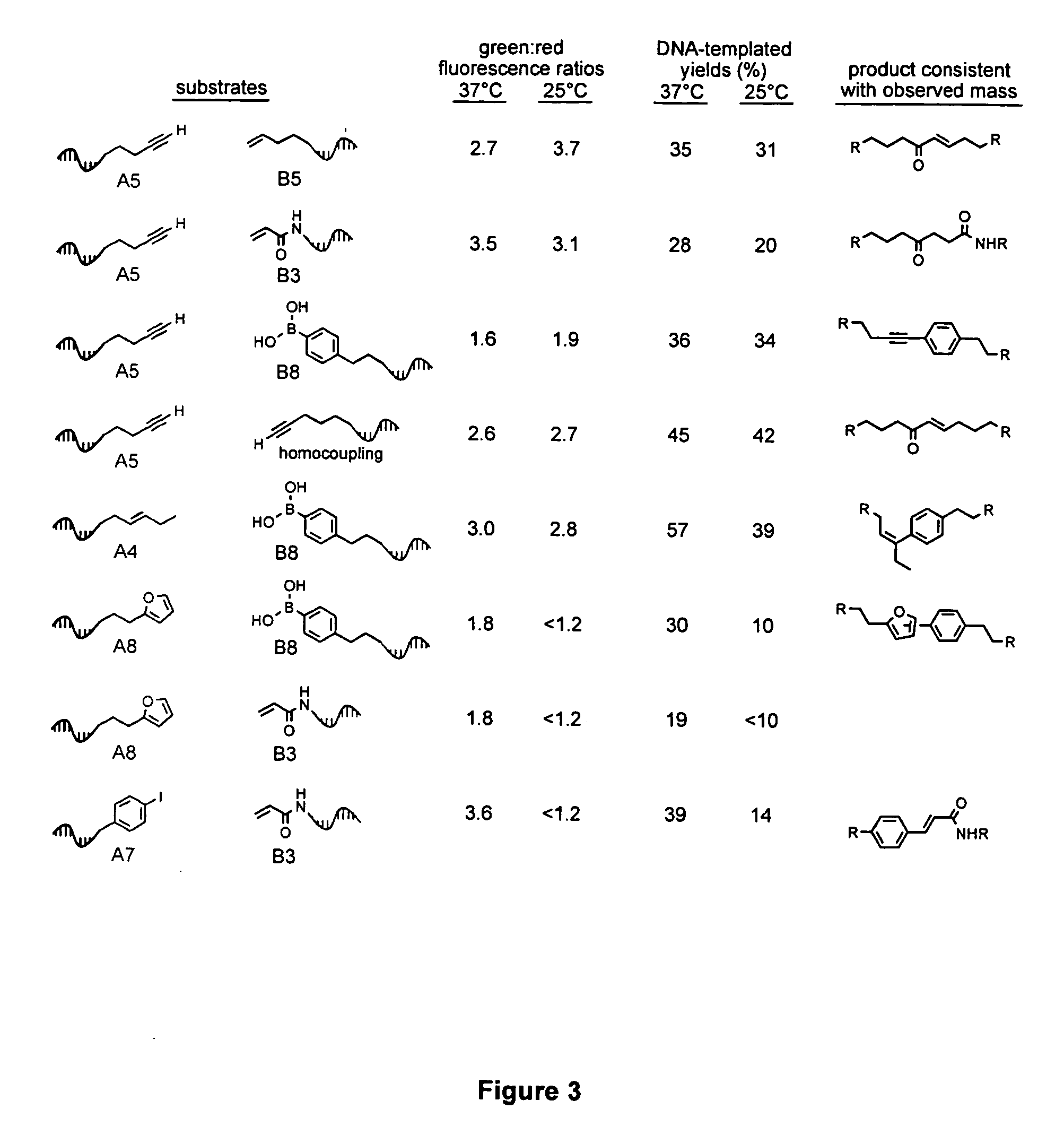
A novel palladium-mediated carbon-carbon bond forming reaction has been discovered using DNA-templated chemistry. The inventive reaction involves the palladium-mediated coupling of a terminal alkyne with an alkene to form an enone. A catalytic amount of palladium may be used in the reaction if an oxidant is present. The reactions is also compatible with a variety of organic solvent as well as aqueous solution. Both intermolecular and intramolecular reactions have been demonstrated. This novel carbon-carbon bond forming reaction is particularly useful in the synthesis of macrocycles. Kits, reagents, catalysts, solvents, oxidants, salts, acids, instructions, and other materials useful in the practice of the inventive reaction are also provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method for preparing 6-hydroxy-2(1H)-quinolone in ionic liquid by using one-pot method
InactiveCN102030706AEasy to useReduce lossesOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAnilineIonic liquid
The invention discloses a method for preparing 6-hydroxy-2(1H)-quinolone in ionic liquid by using a one-pot method. The invention synthesizes the 6-hydroxy-2(1H)-quinolone by through bromination, acidation and intramolecular Heck reaction of p-methoxy aniline and acryloyl chloride as raw materials in the ionic liquid by using the one-pot method. The method has simple synthesis line, low product cost and high yield, is environmental friendly and is easy to realize industrial production.
Owner:JIANGSU FOOD SCI COLLEGE
Polymer precursor, high transparency polyimide precursor, polymer compound, resin composition and article using thereof
ActiveUS20060229384A1Low thermal expansionLow moisture absorptionMagnetic materials for record carriersOptical filtersLength waveSingle bond
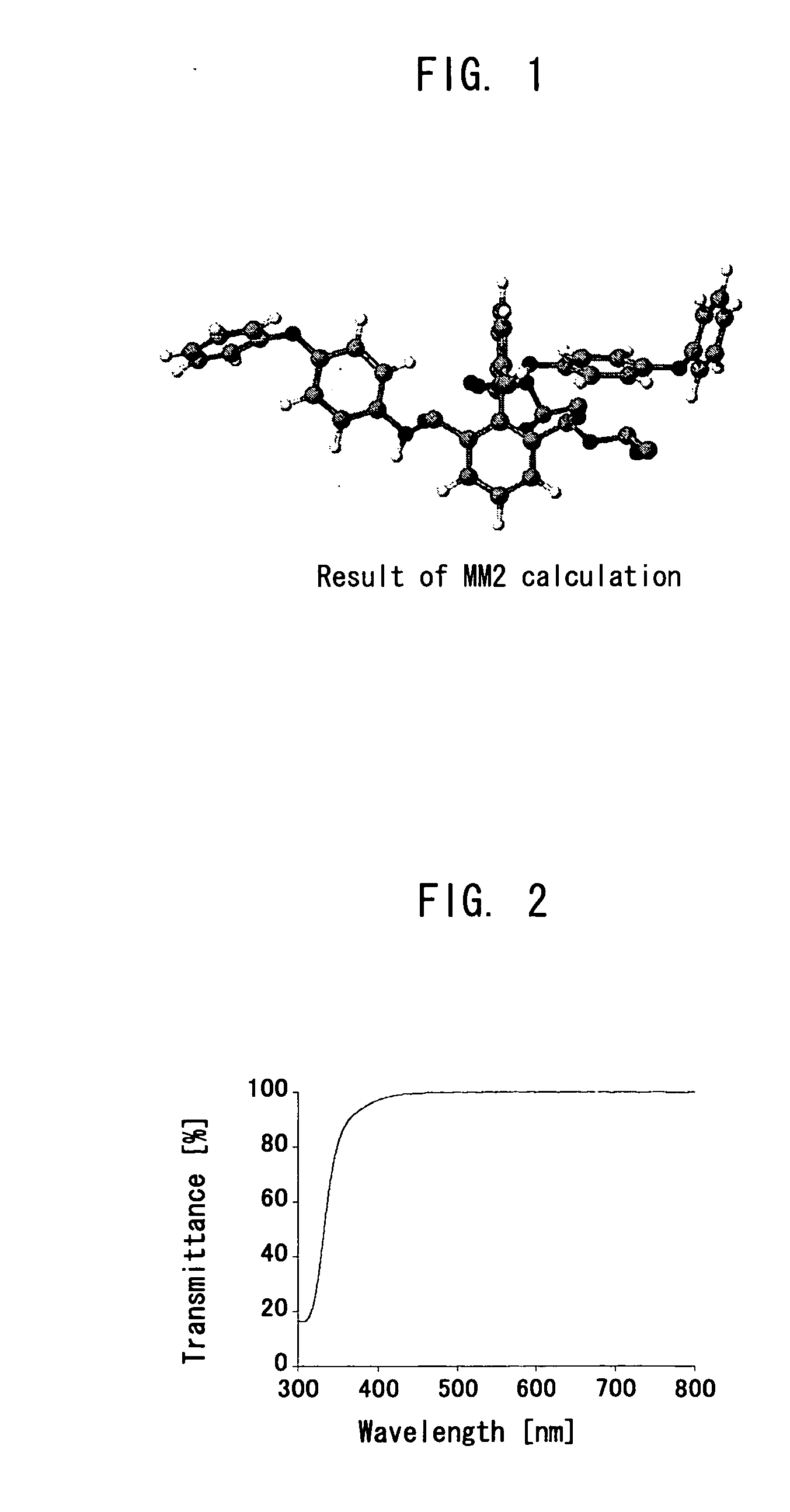
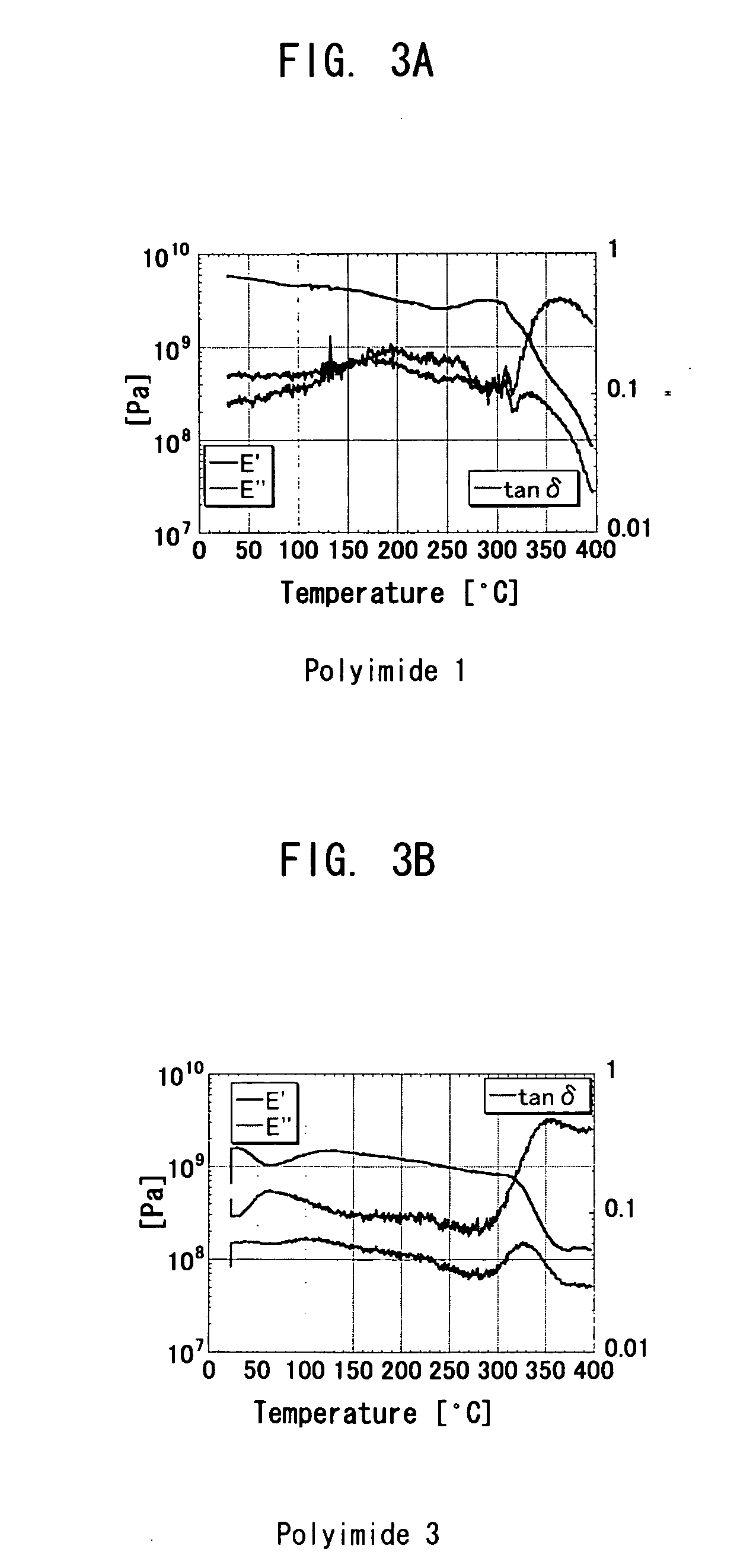
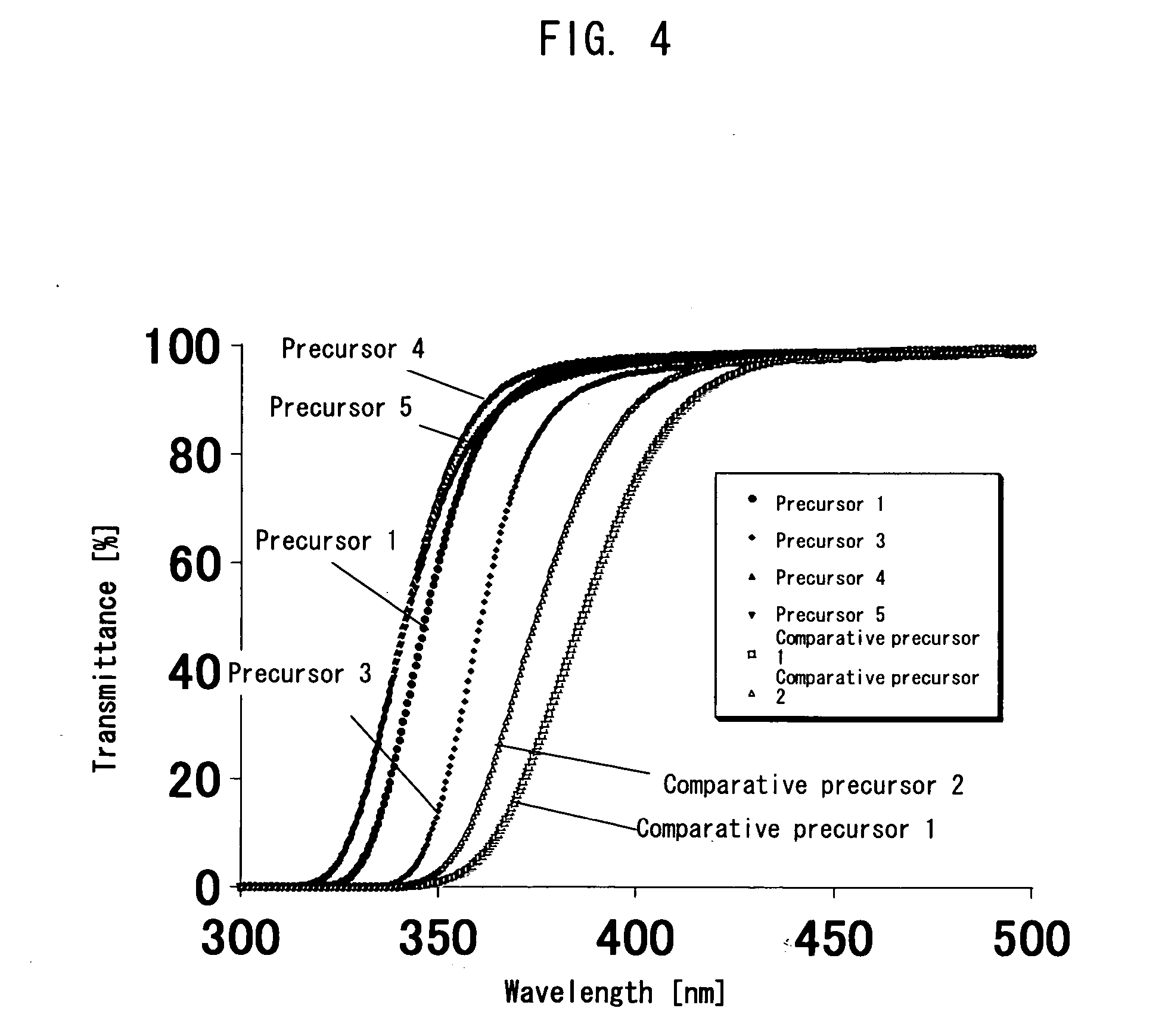
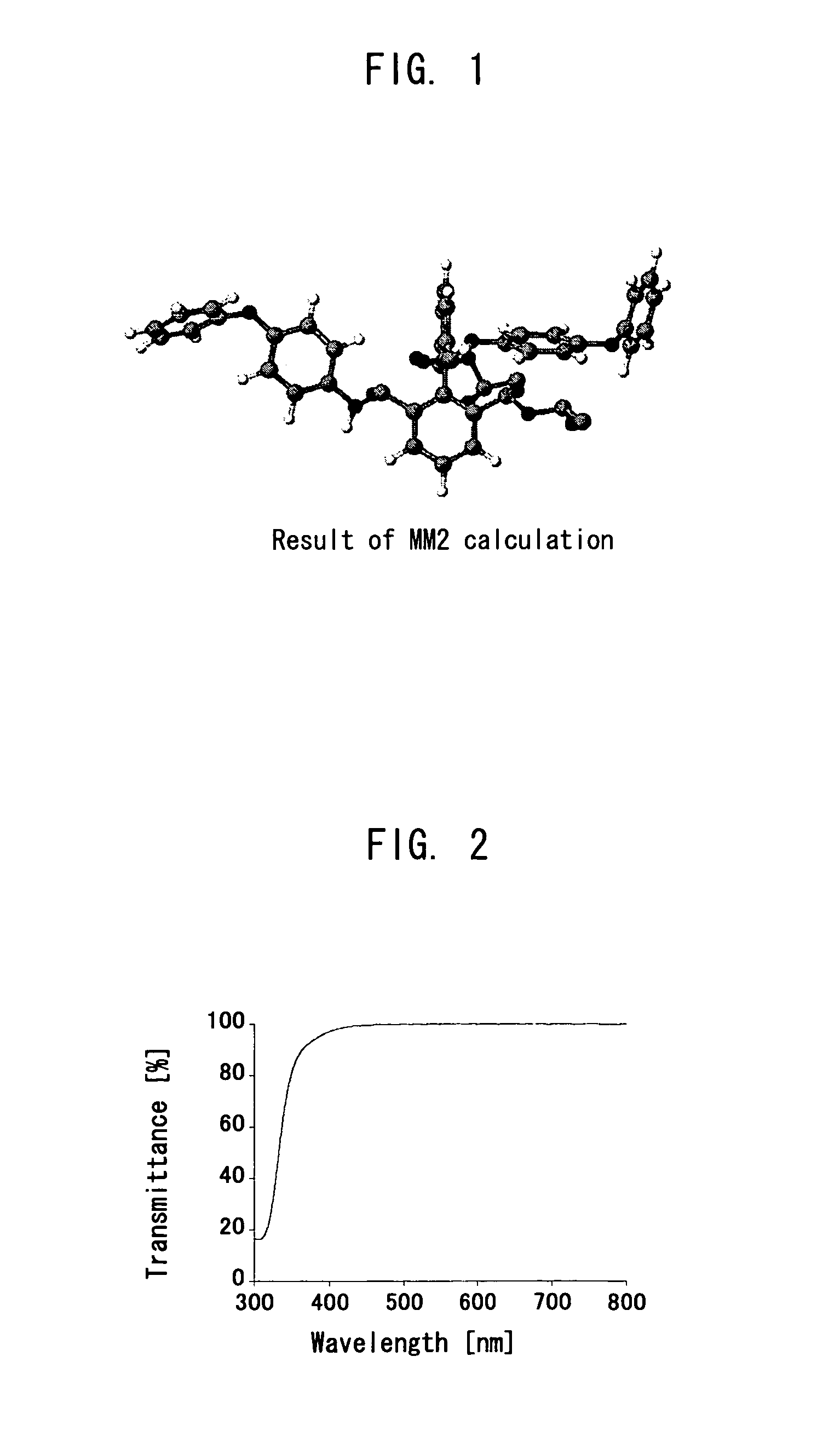
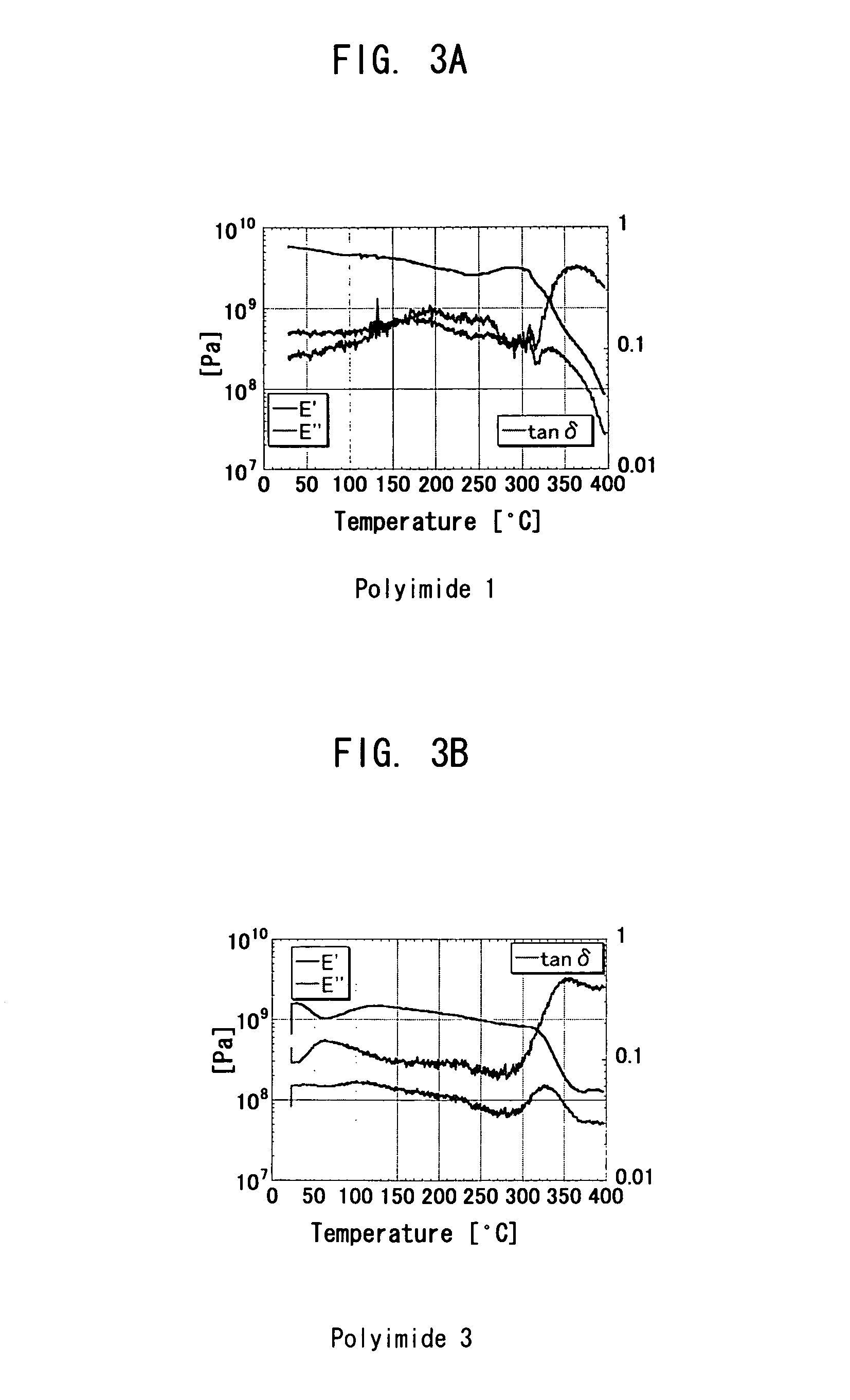
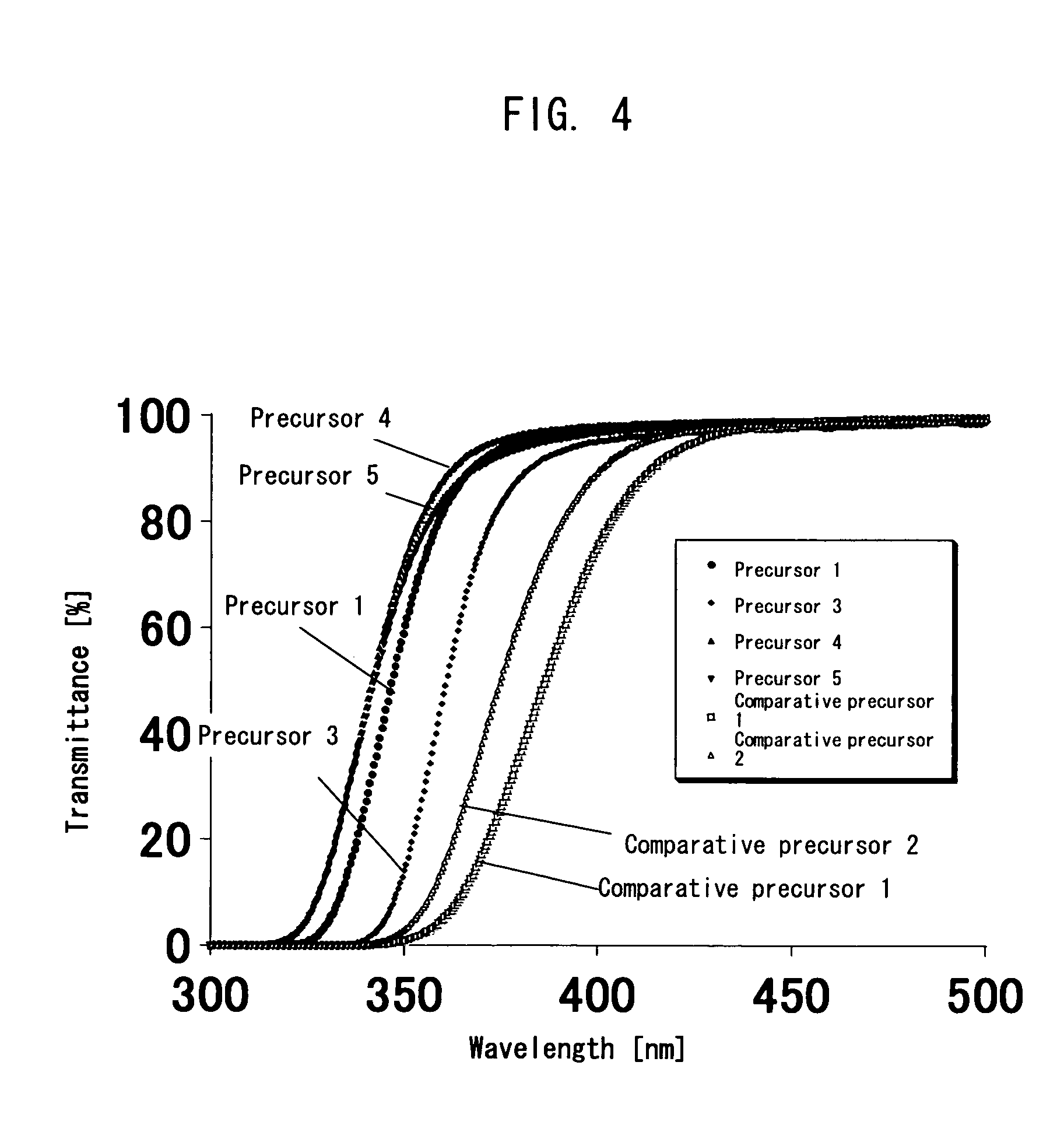
A main object of the present invention is to provide a polymer precursor which exhibits high transmittance to a shorter wavelength range with respect to an electromagnetic wave though the polymer precursor has a part which sequences an unsaturated bond having a π electron orbit and a single bond alternately. In order to attain the object, a polymer precursor comprising a part which sequences an unsaturated bond having a π electron orbit and a single bond alternately, wherein the polymer precursor has a first functional group and a second functional group which form a repeating unit constituting a polymer skeleton of an end product by an intramolecular reaction, wherein at least a part of a conjugated state formed by the π electron orbit in the molecule is disconnected or weakened due to a three-dimensional structure of the molecule, and wherein a transmittance with respect to an electromagnetic wave of at least one wavelength selected from the group consisting of 436 nm, 405 nm, 365 nm, 248 nm and 193 nm is improved, is provided.
Owner:DAI NIPPON PRINTING CO LTD
Polymer precursor, high transparency polyimide precursor, polymer compound, resin composition and article using thereof
ActiveUS8088882B2High sensitivityGood storage stabilityMagnetic materials for record carriersOptical filtersLength waveSingle bond
A main object of the present invention is to provide a polymer precursor which exhibits high transmittance to a shorter wavelength range with respect to an electromagnetic wave though the polymer precursor has a part which sequences an unsaturated bond having a π electron orbit and a single bond alternately. In order to attain the object, a polymer precursor comprising a part which sequences an unsaturated bond having a π electron orbit and a single bond alternately, wherein the polymer precursor has a first functional group and a second functional group which form a repeating unit constituting a polymer skeleton of an end product by an intramolecular reaction, wherein at least a part of a conjugated state formed by the π electron orbit in the molecule is disconnected or weakened due to a three-dimensional structure of the molecule, and wherein a transmittance with respect to an electromagnetic wave of at least one wavelength selected from the group consisting of 436 nm, 405 nm, 365 nm, 248 nm and 193 nm is improved, is provided.
Owner:DAI NIPPON PRINTING CO LTD
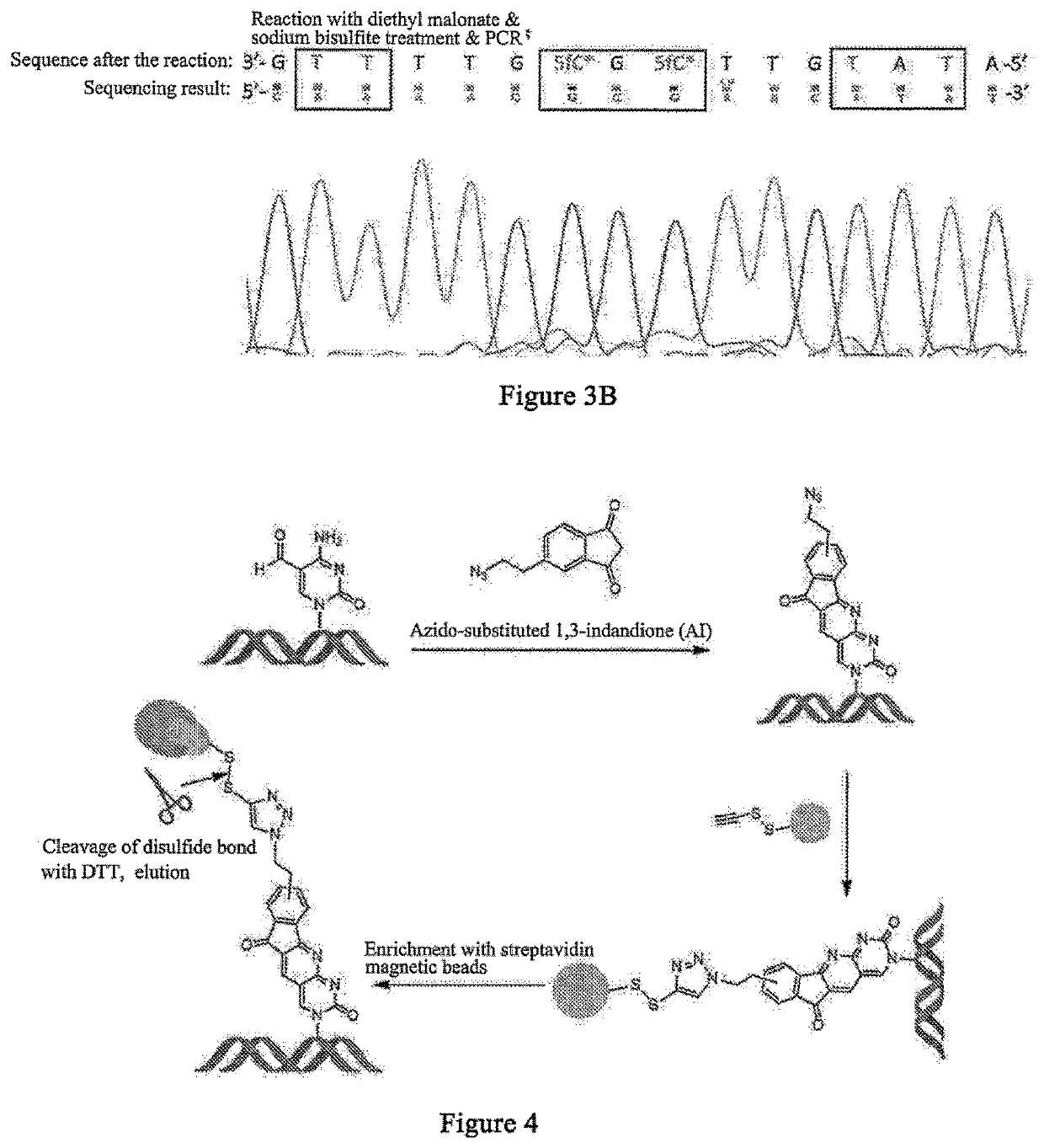
5-formylcytosine specific chemical labeling method and related applications
ActiveUS20160362438A1Achieve conversionHigh selectivitySugar derivativesMicrobiological testing/measurementChemical labelingCytosine
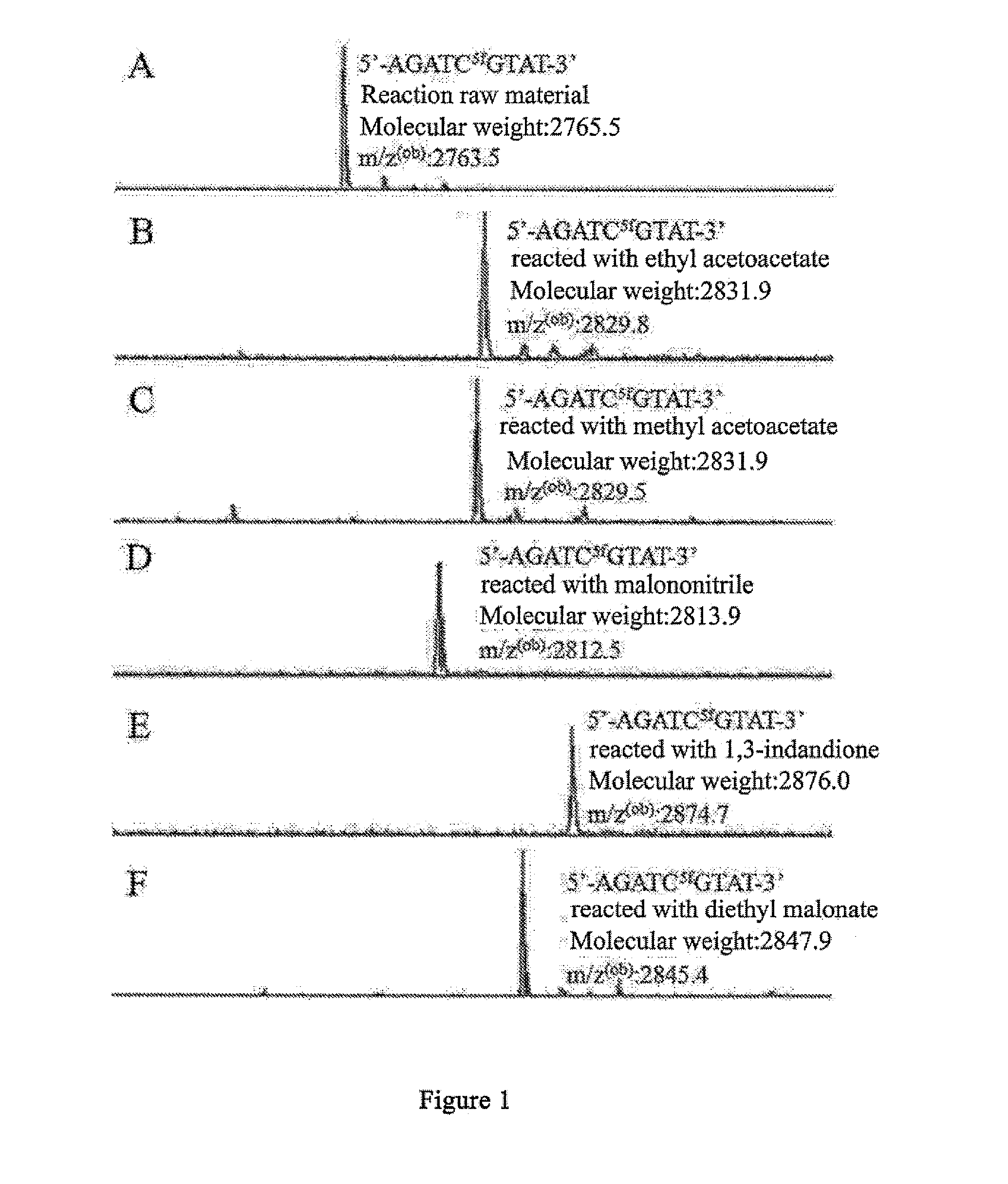
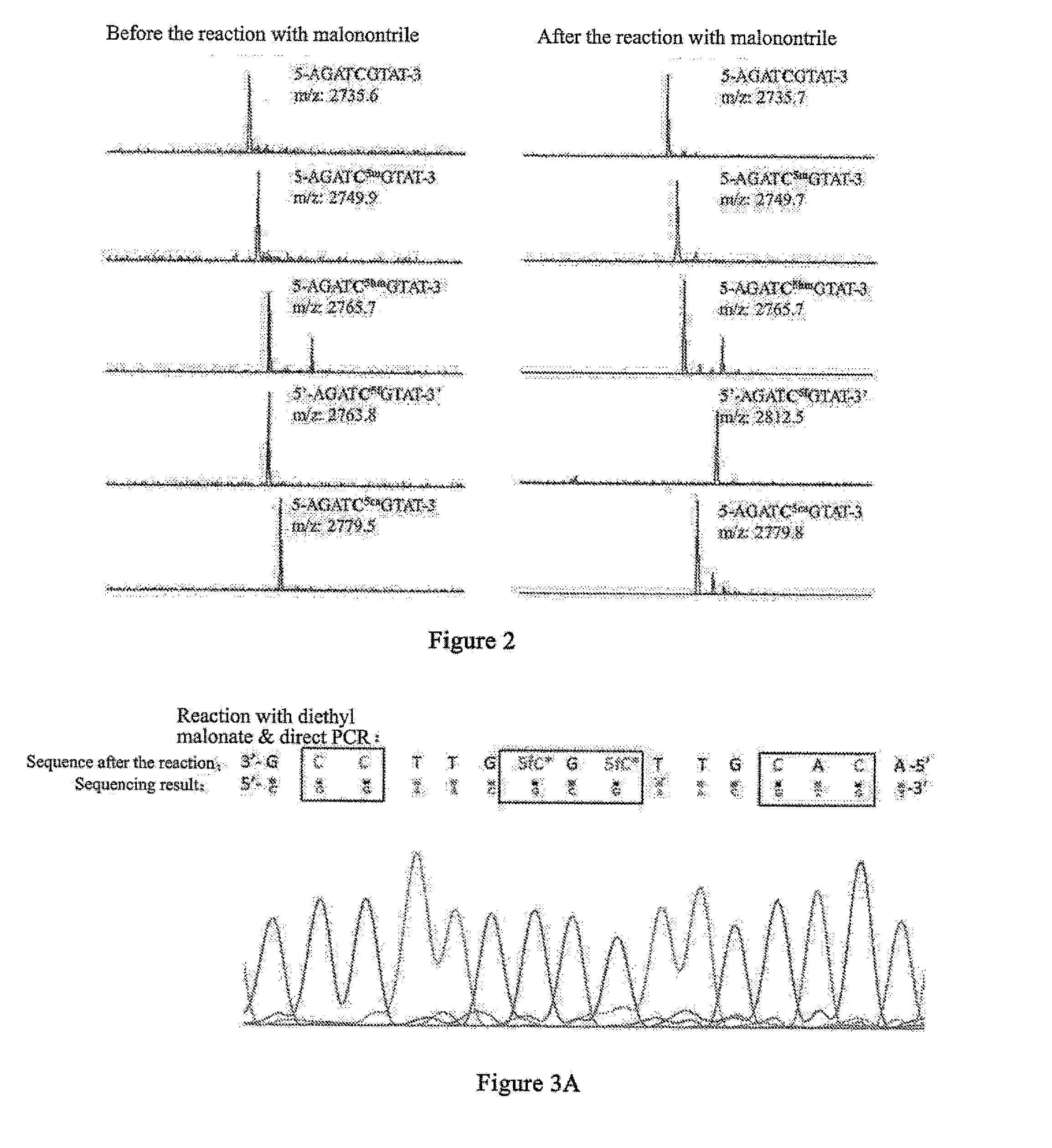
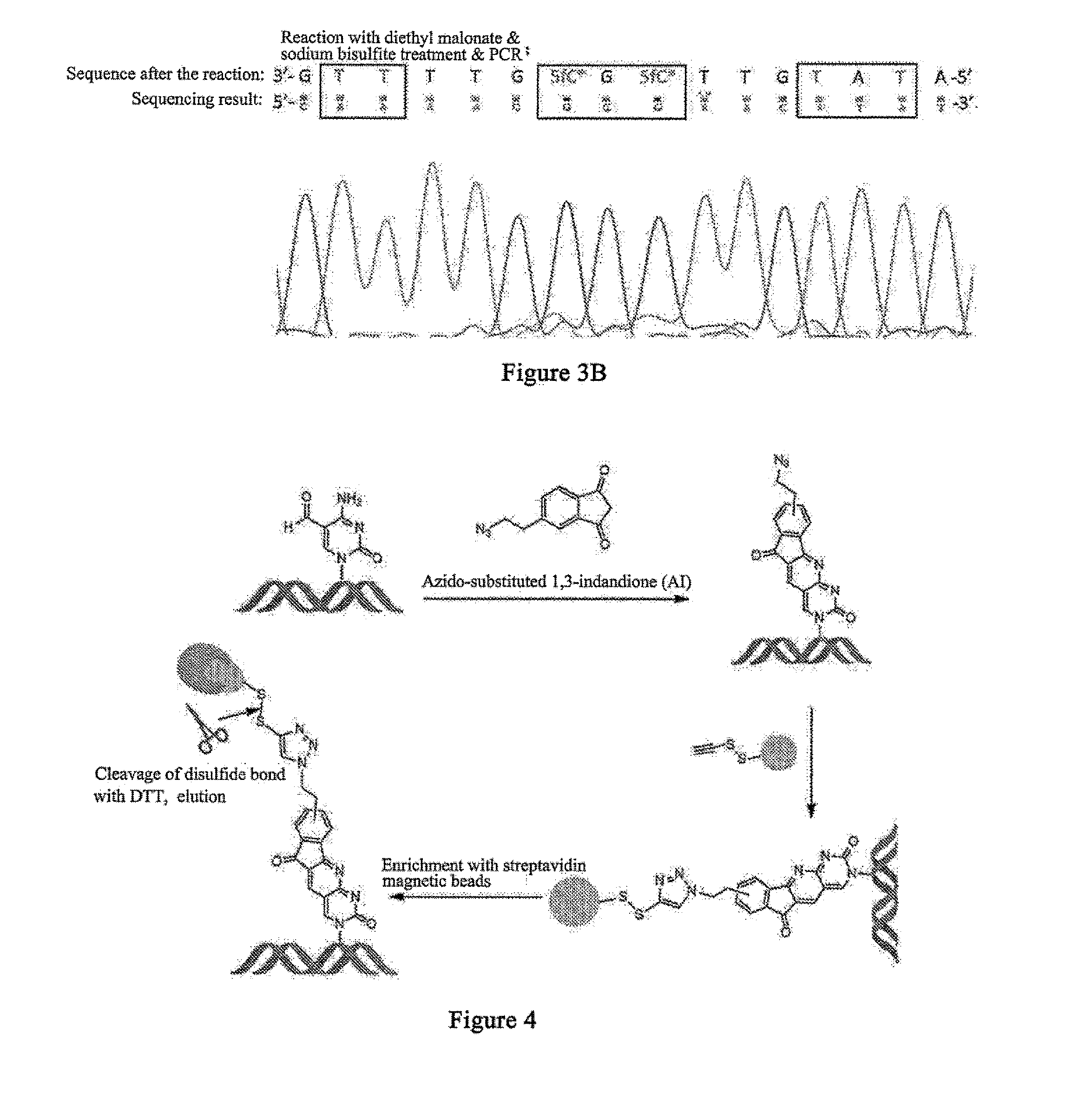
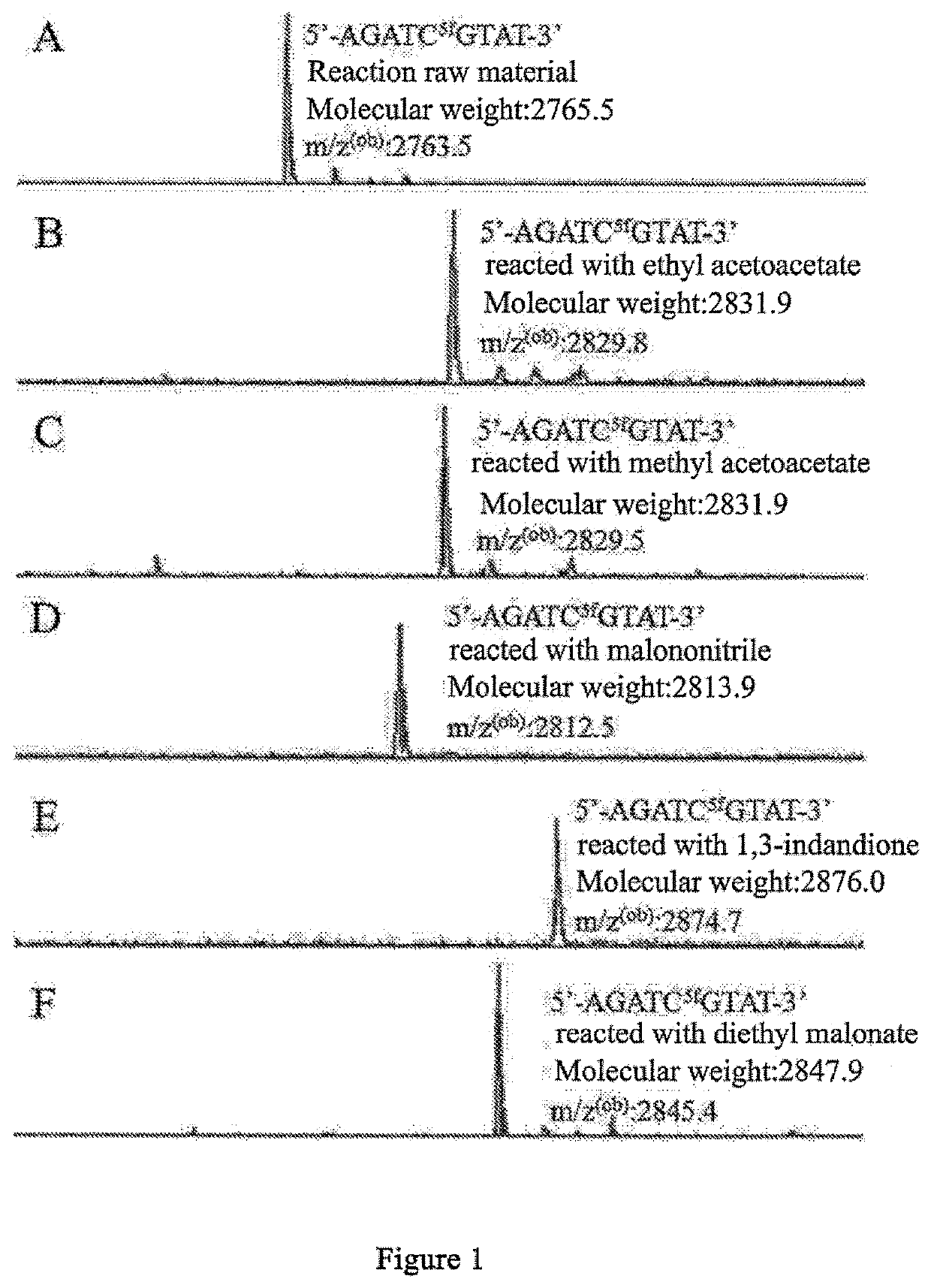
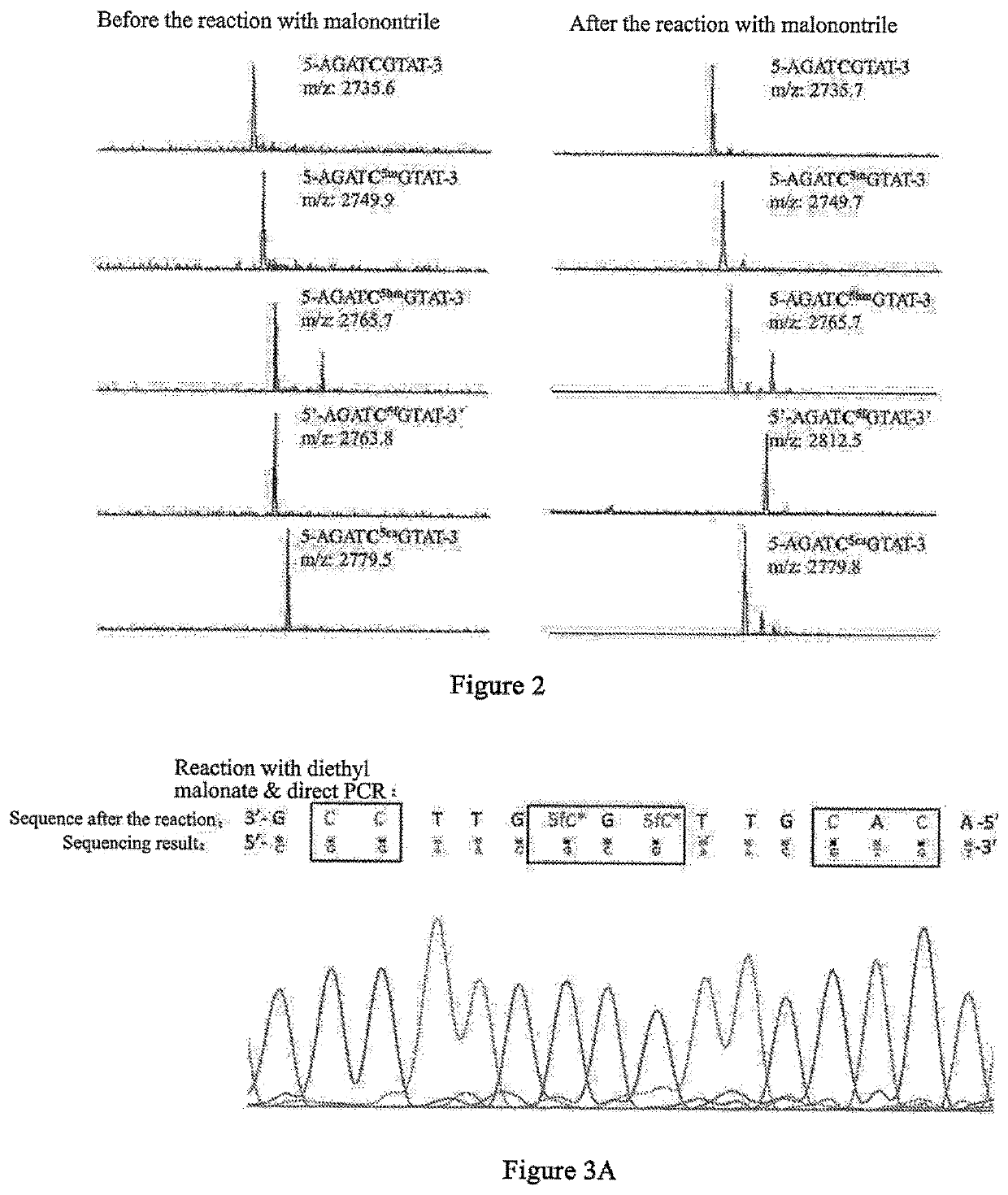
The present invention relates to a 5-formylcytosine specific chemical labeling method and related applications in aspects such as sequencing, detection, imaging, and diagnosis. In the method, a condensation reaction occurs between an active methylene group in an active methylene compound containing a side-chain reactive group and an aldehyde group in 5-formylcytosine or a 1-substituted derivative of 5-formylcytosine, and at the same time an intramolecular reaction occurs between the side-chain reactive group of the active methylene compound and a 4-amino group of cytosine to implement ring closing. By means of the 5-formylcytosine specific chemical labeling method and related compounds of the present invention, detection of the content of 5-formylcytosine in nucleic acid molecules, and specific concentration of 5-formylcytosine-containing nucleic acid samples, and analysis of sequence distribution information of 5-formylcytosine and / or single-base resolution sequence information in nucleic acid molecules and the like may be implemented. The present invention provides various effective research methods in the research fields of epigenetics and nucleic acid biochemistry.
Owner:PEKING UNIV
Sustainable chemical processes
InactiveUS20070032385A1Organic chemistrySuperconductor device manufacture/treatmentCompound (substance)Structural formula
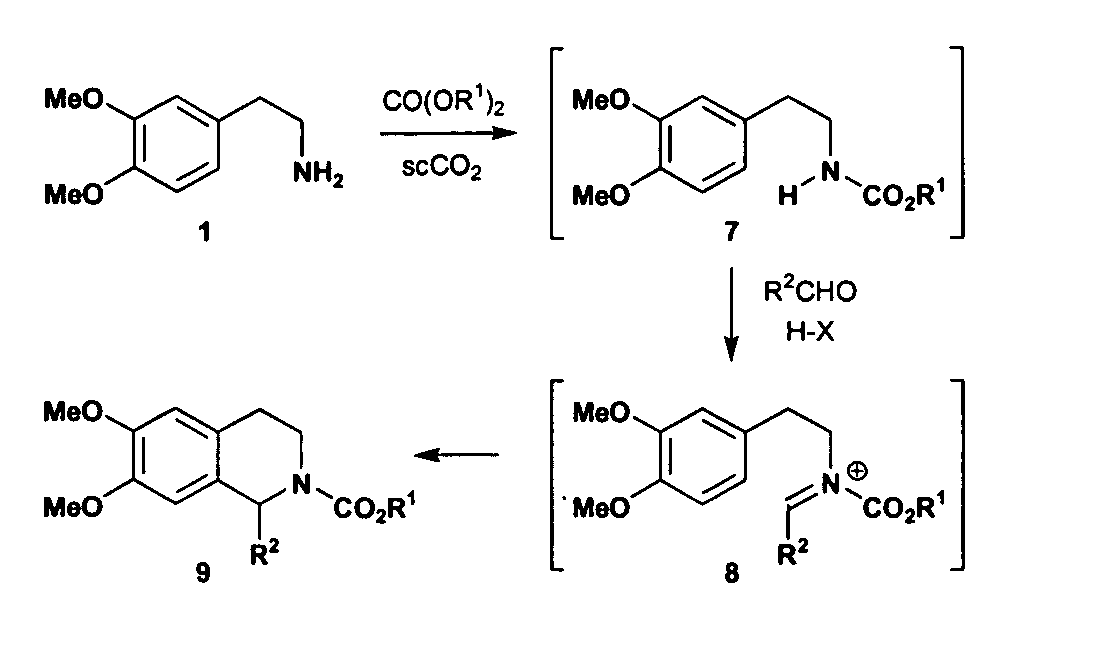
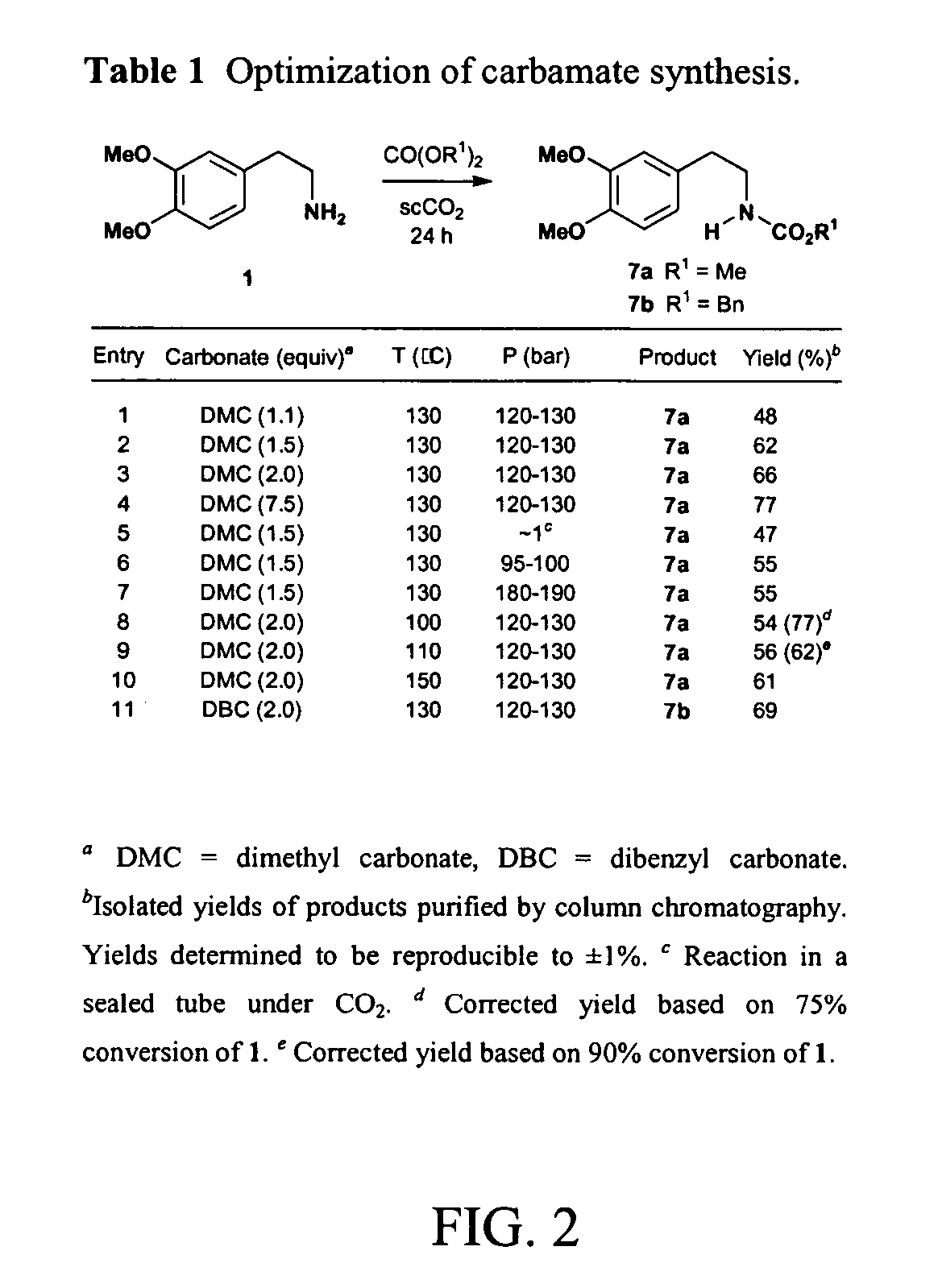
A method includes preparing a protected amine compound represented by the following structural formula: wherein the dotted line - - - is a covalent bond or no bond. The method includes the step of, in the presence of superatmospheric CO2: a) intermolecularly reacting an iminium compound with a nucleophile Nu represented by the following structural diagram: or b) intramolecularly reacting an iminium group of an iminium compound represented by the following structural formula, the iminium compound having a nucleophile: with the nucleophile of the iminium compound, thereby forming the protected amine compound.
Owner:MASSACHUSETTS INST OF TECH
Palladium-catalyzed carbon-carbon bond forming reactions
ActiveUS20070066851A1High yieldFunction increaseOrganic compound preparationCarboxylic acid amides preparationCarbon–carbon bondAlkyne
A novel palladium-mediated carbon-carbon bond forming reaction has been discovered using DNA-templated chemistry. The inventive reaction involves the palladium-mediated coupling of a terminal alkyne with an alkene to form an enone. A catalytic amount of palladium may be used in the reaction if an oxidant is present. The reactions is also compatible with a variety of organic solvent as well as aqueous solution. Both intermolecular and intramolecular reactions have been demonstrated. This novel carbon-carbon bond forming reaction is particularly useful in the synthesis of macrocycles. Kits, reagents, catalysts, solvents, oxidants, salts, acids, instructions, and other materials useful in the practice of the inventive reaction are also provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
5-formylcytosine specific chemical labeling method and related applications
ActiveUS10519184B2High selectivityGood fluorescence propertiesSugar derivativesMicrobiological testing/measurementChemical labelingSide chain
The present invention relates to a 5-formylcytosine specific chemical labeling method and related applications in aspects such as sequencing, detection, imaging, and diagnosis. In the method, a condensation reaction occurs between an active methylene group in an active methylene compound containing a side-chain reactive group and an aldehyde group in 5-formylcytosine or a 1-substituted derivative of 5-formylcytosine, and at the same time an intramolecular reaction occurs between the side-chain reactive group of the active methylene compound and a 4-amino group of cytosine to implement ring closing. By means of the 5-formylcytosine specific chemical labeling method and related compounds of the present invention, detection of the content of 5-formylcytosine in nucleic acid molecules, and specific concentration of 5-formylcytosine-containing nucleic acid samples, and analysis of sequence distribution information of 5-formylcytosine and / or single-base resolution sequence information in nucleic acid molecules and the like may be implemented. The present invention provides various effective research methods in the research fields of epigenetics and nucleic acid biochemistry.
Owner:PEKING UNIV
Method for preparing anidulafungin
InactiveCN103145809AHigh yieldEasy post-processingPeptide preparation methodsBulk chemical productionFluid phaseCombinatorial chemistry
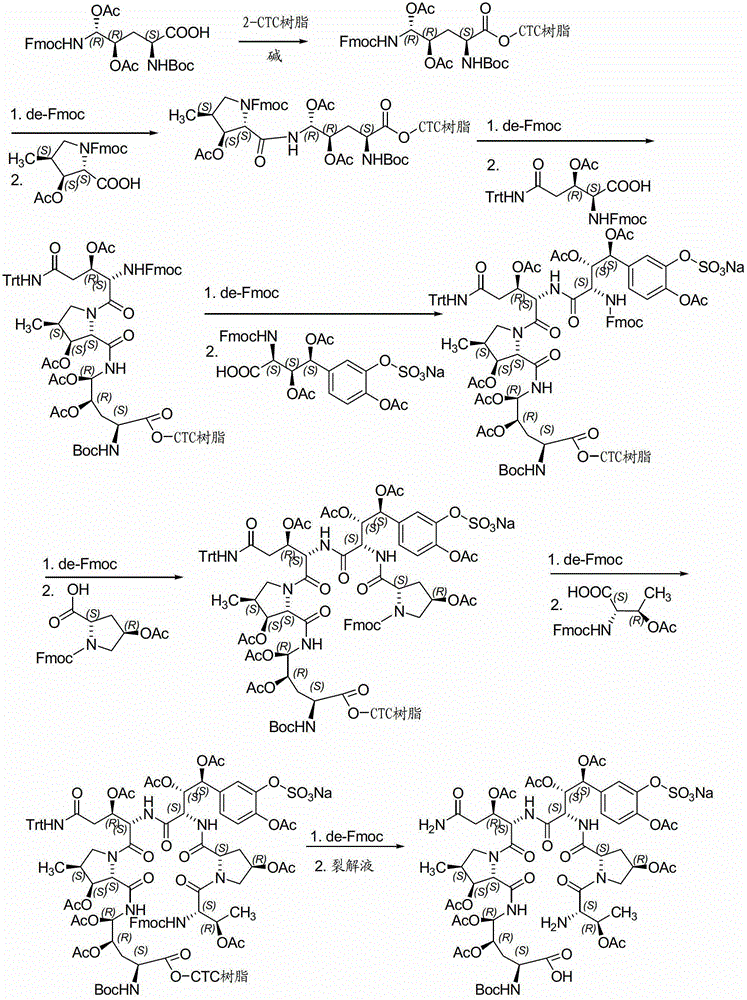
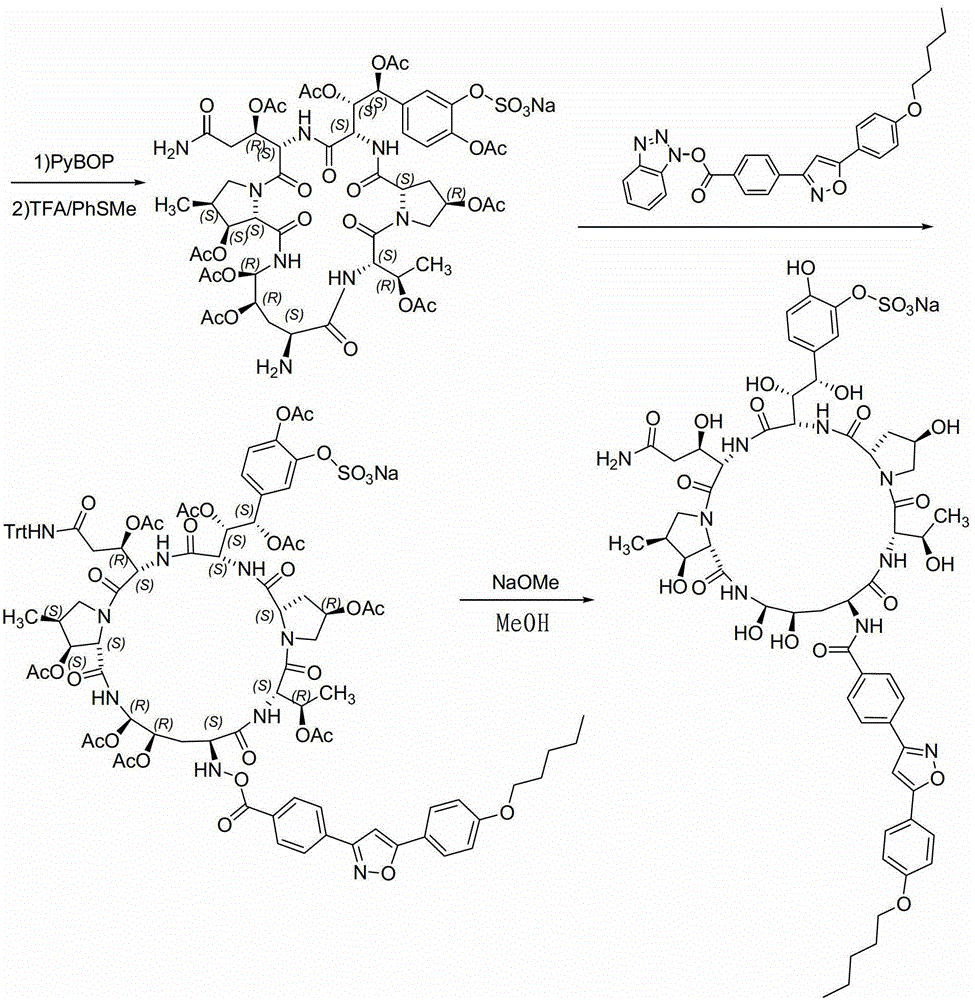
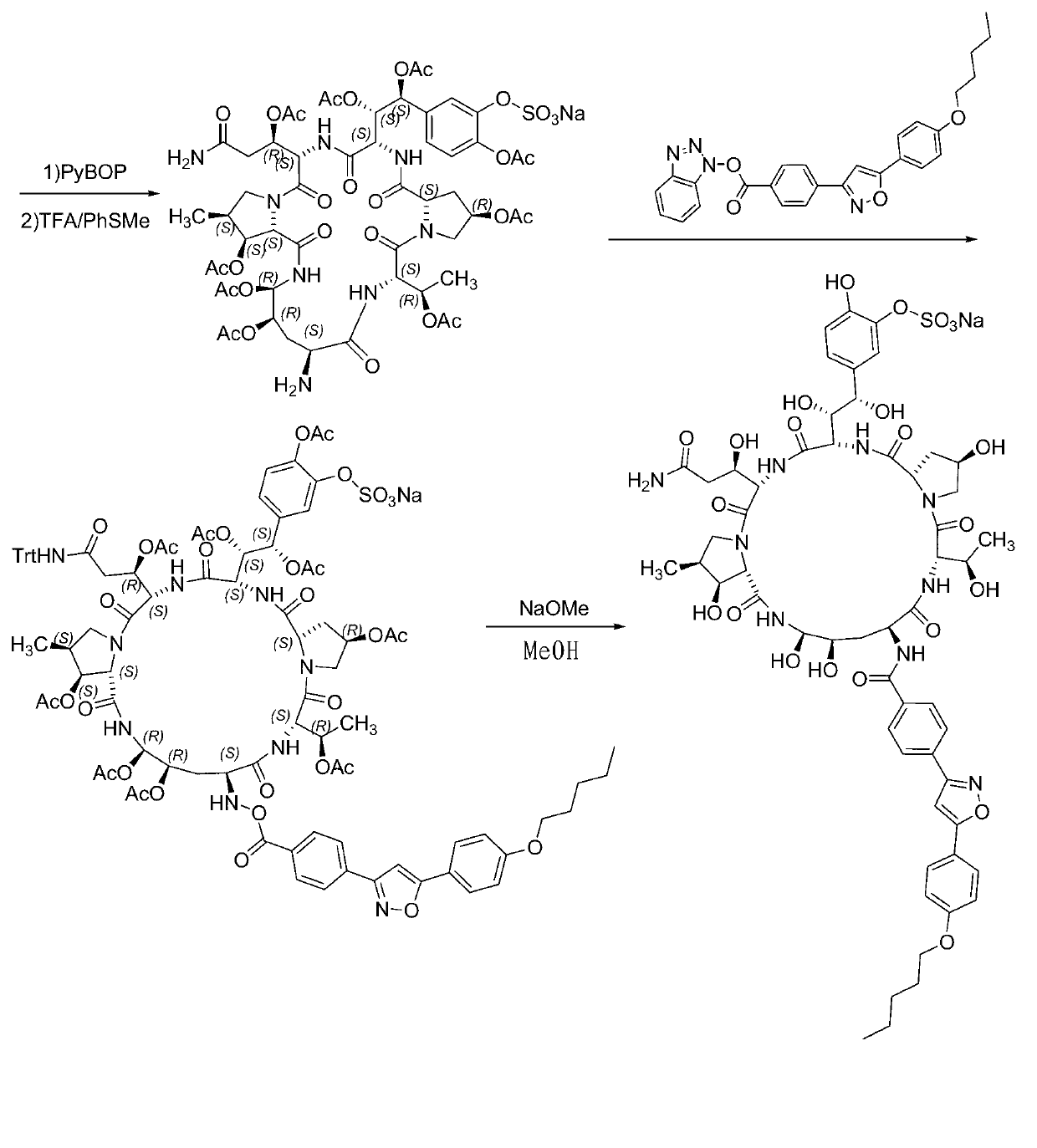
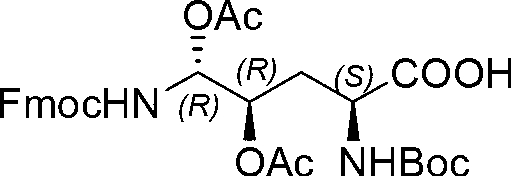
The invention relates to a solid phase-liquid phase full synthetic method for preparing anidulafungin. The method comprises that Fmoc-P-resin serves as a carrier, a solid phase polypeptide synthetic method is adopted to gradually couple amino acid with an Fmoc protecting group from the C end to the N end, and then the protecting group is deprived through splitting, intramolecular reaction and liquid phase condensation to obtain the anidulafungin. The method is high in yield, brings convenience to aftertreatment and provides a new thinking way for industrial scale production of the anidulafungin.
Owner:HYBIO PHARMA
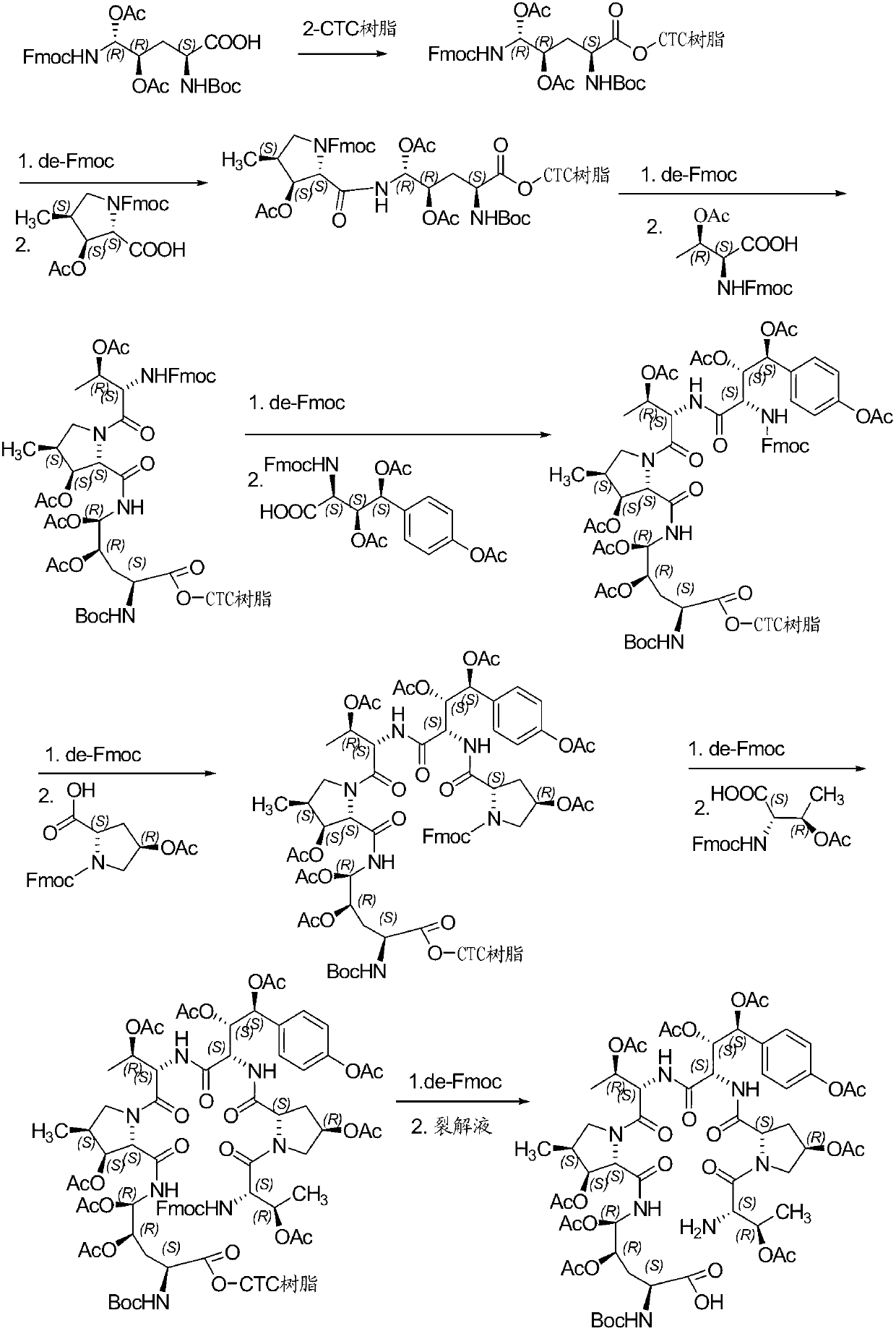
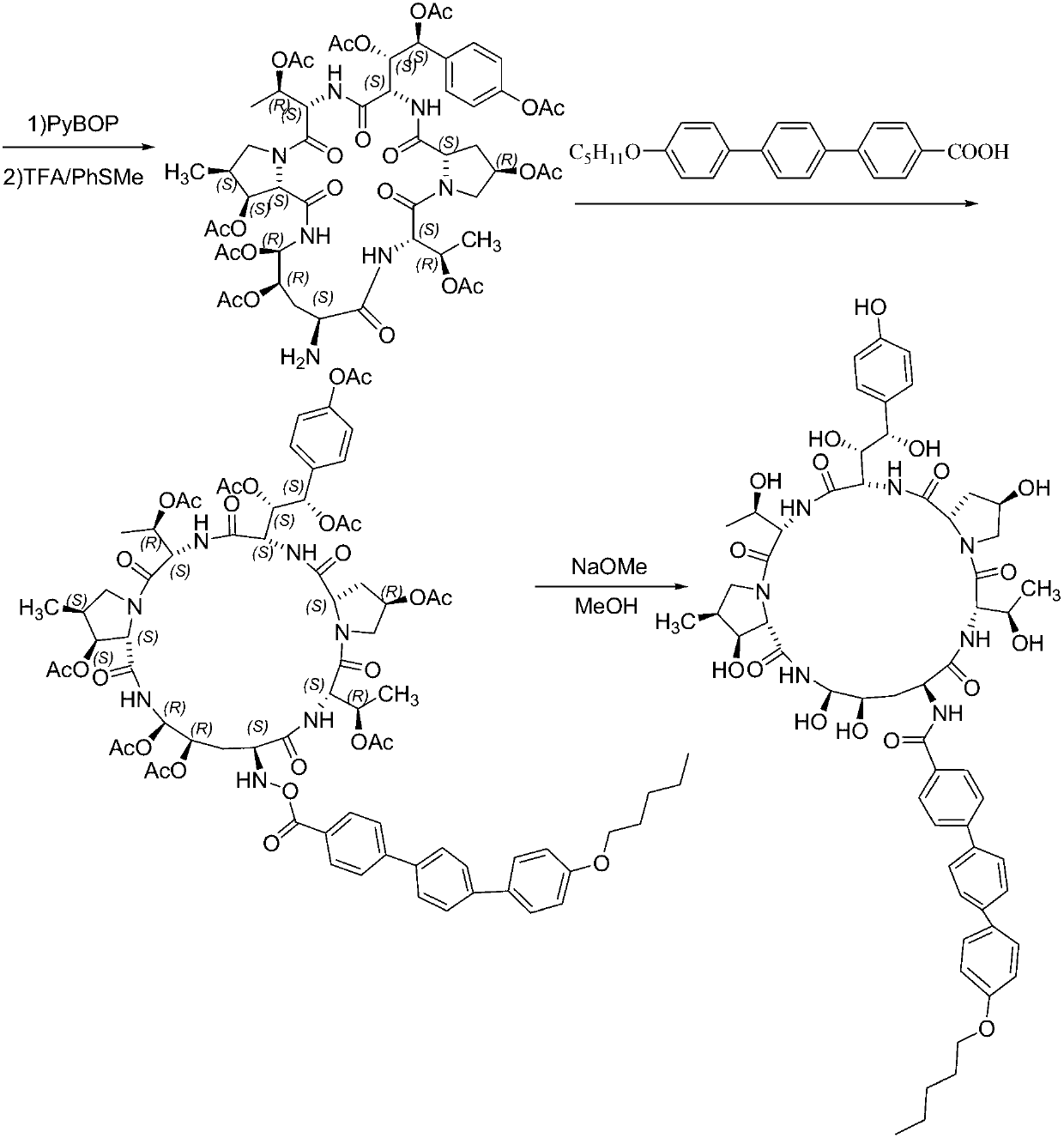
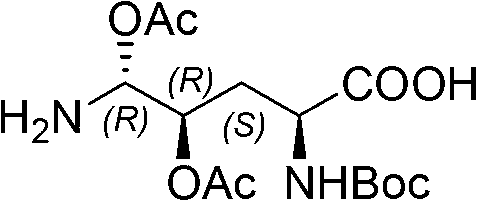
A kind of method for preparing micafungin
InactiveCN103145810BHigh yieldEasy post-processingPeptide preparation methodsBulk chemical productionMicafunginProtecting group
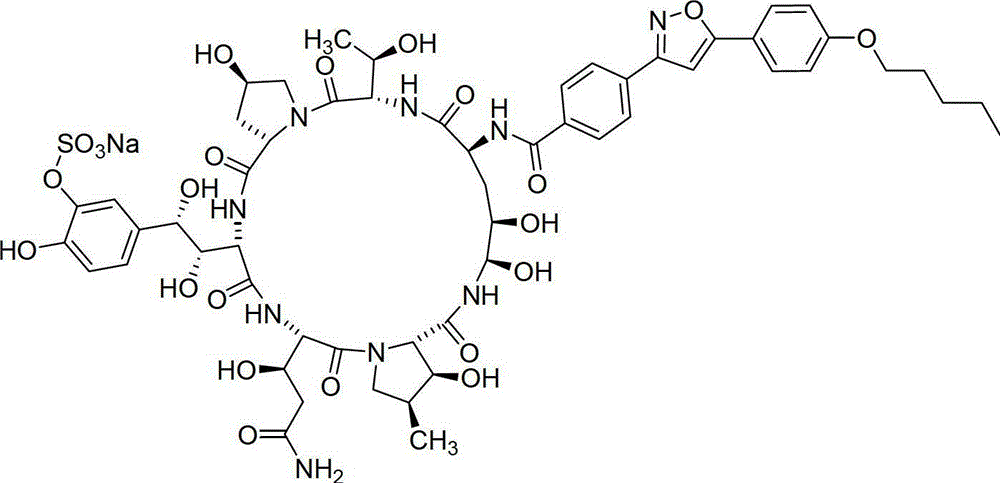
The invention relates to a solid phase-liquid phase full synthetic method for preparing micafungin. The method comprises that Fmoc-P-CTC resin serves as a carrier, a solid phase polypeptide synthetic method is adopted to gradually couple amino acid with an Fmoc protecting group from the C end to the N end, and then the protecting group is deprived through splitting, intramolecular reaction and liquid phase condensation to obtain the micafungin. The method is high in yield, brings convenience to aftertreatment and provides a new thinking way for industrial scale production of the micafungin.
Owner:HYBIO PHARMA
Novel total synthesis method of racemic tetrandrine
ActiveCN113045578AMaximize utilizationHigh synthesis efficiencyOrganic chemistryBulk chemical productionChemical synthesisPhenylacetic acid
The invention discloses a novel total synthesis method of racemic tetrandrine, and belongs to the technical field of pharmaceutical chemical synthesis. 5-bromovanillin and 4-hydroxyphenylacetic acid which are low in price and easy to obtain are respectively used as starting materials to synthesize a compound 10, the compound 10 is used for synthesizing a compound 12 and a compound 14 through a simple route, and the two compounds are subjected to intermolecular and intramolecular Ullmann reaction to synthesize the racemic tetrandrine A. The key intermediates 12 and 14 are synthesized through the compound 10, the synthesis efficiency is greatly improved, the raw materials are utilized to the maximum extent, and the method is a racemic tetrandrine total synthesis route which is simplest in operation and lowest in cost so far.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD +1
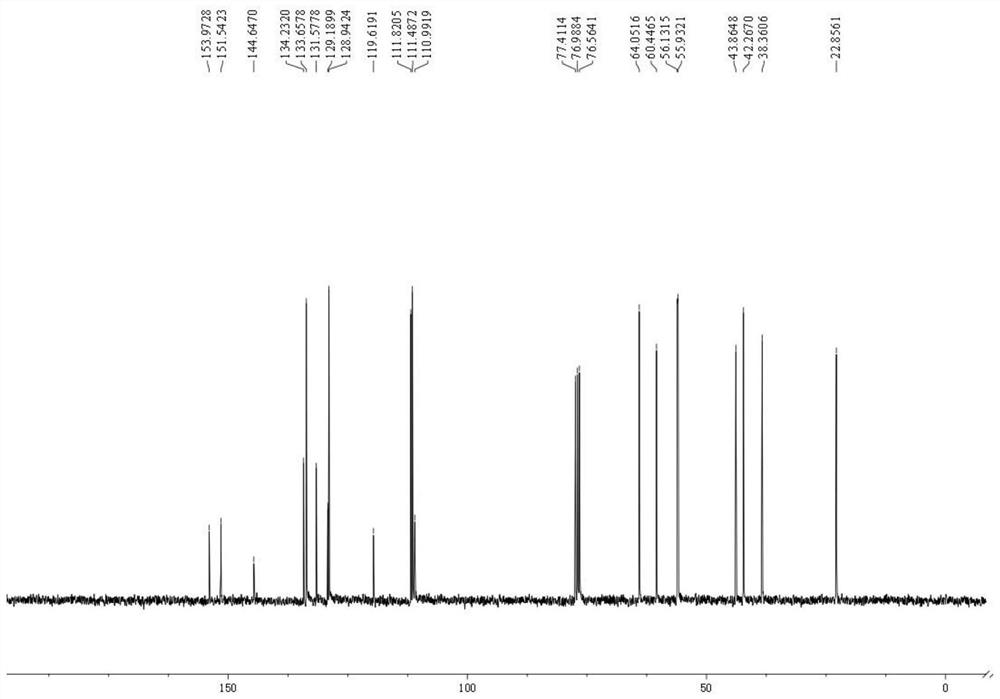
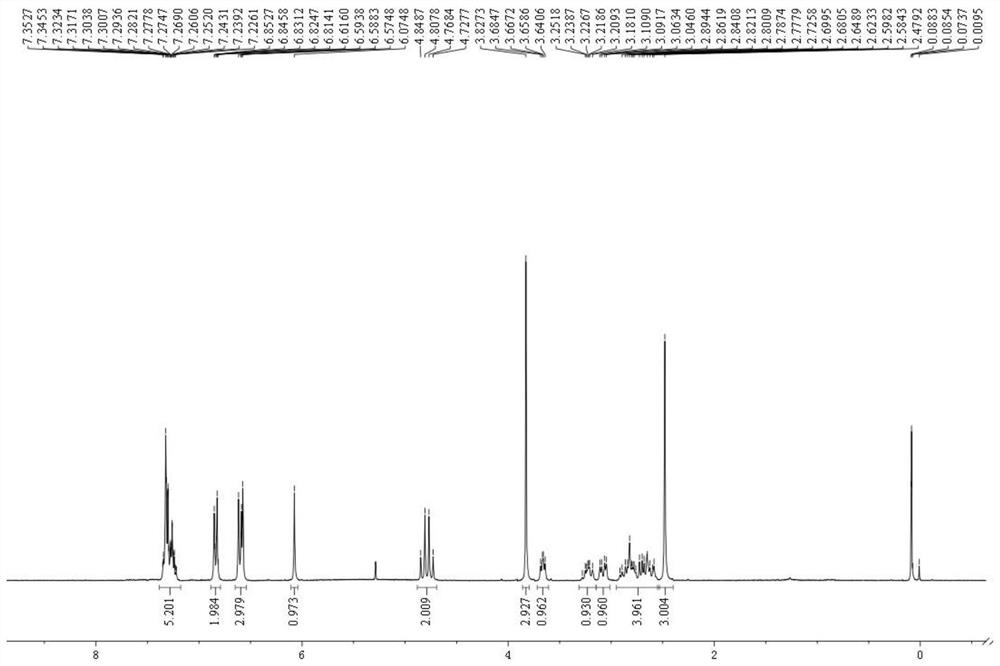
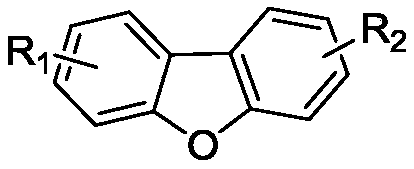
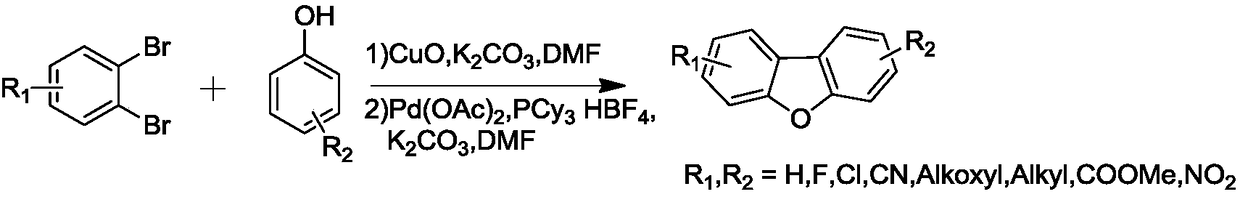
Dibenzofuran derivative and preparation method thereof
The invention belongs to the technical field of organic optoelectronic materials, and particularly relates to a dibenzofuran derivative and a preparation method thereof. A one-pot method is used for dissolving dibromobenzene and substituted phenol into an organic solvent; backflow reaction is performed for 10 hours; then, intramolecular reaction is performed under the effect of catalyst effects, so as to obtain; the substituted dibenzofuran derivative can be obtained atwith the yield of 80 percent. The method provided by the invention has the advantages that the raw material sources are wide;, and economical performance is realized; the operation is simple and convenient; the product can be easily separated and purified. In addition, the substituted dibenzofuran derivative related byin the invention has the advantages that the multi-functional group substituted products can be simply obtained; the net next derivation can be performed for synthesizing a series of materials based ontaking dibenzofuran as mother bodies; the kind type of material has high power supply performance and conjugacy performance, and is hopeful to be used in the field of an organic photoelectric material.
Owner:XIAN MODERN CHEM RES INST
Heterocyclic organic molecules through intramolecular formation of n-acyliminium ions
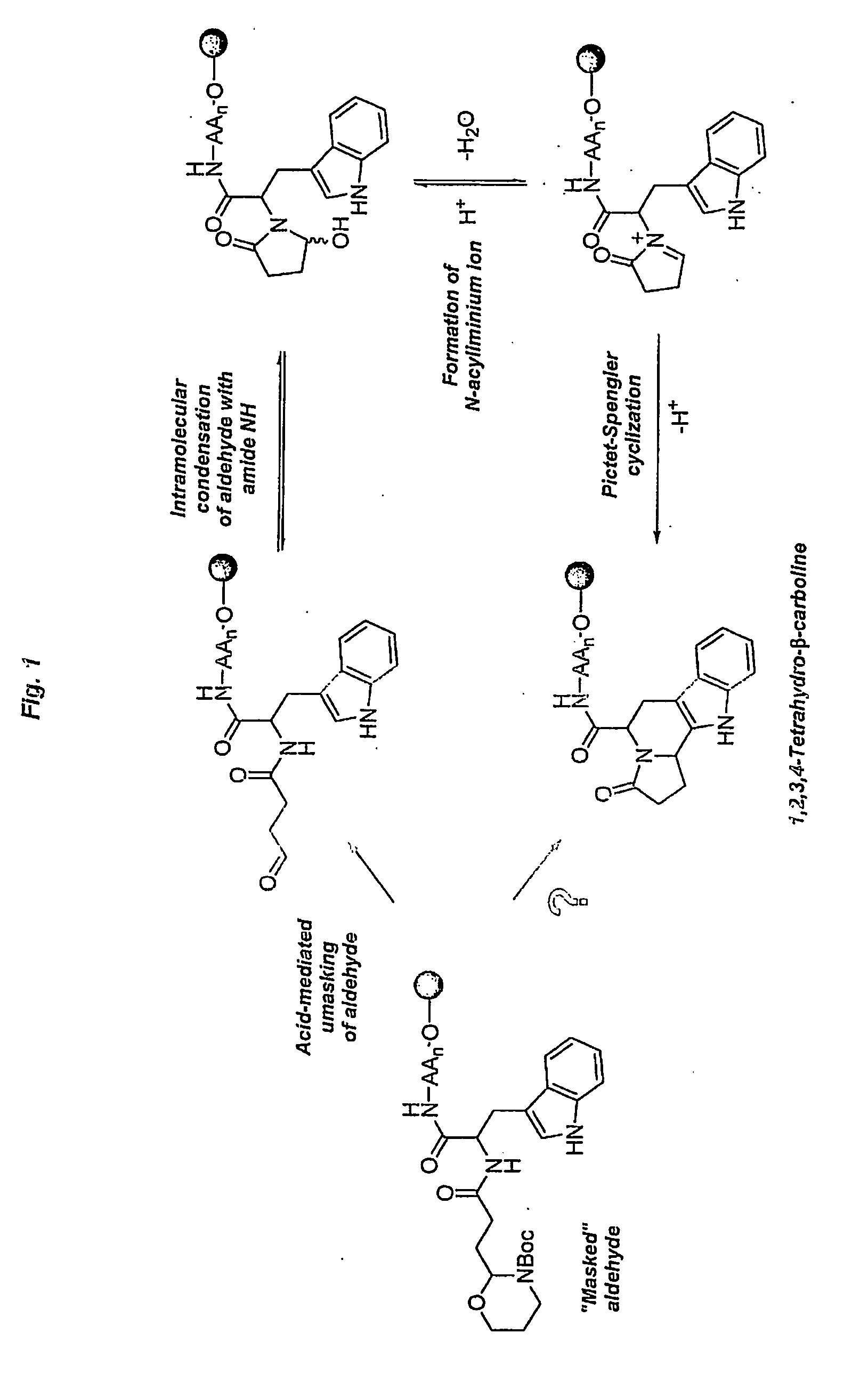
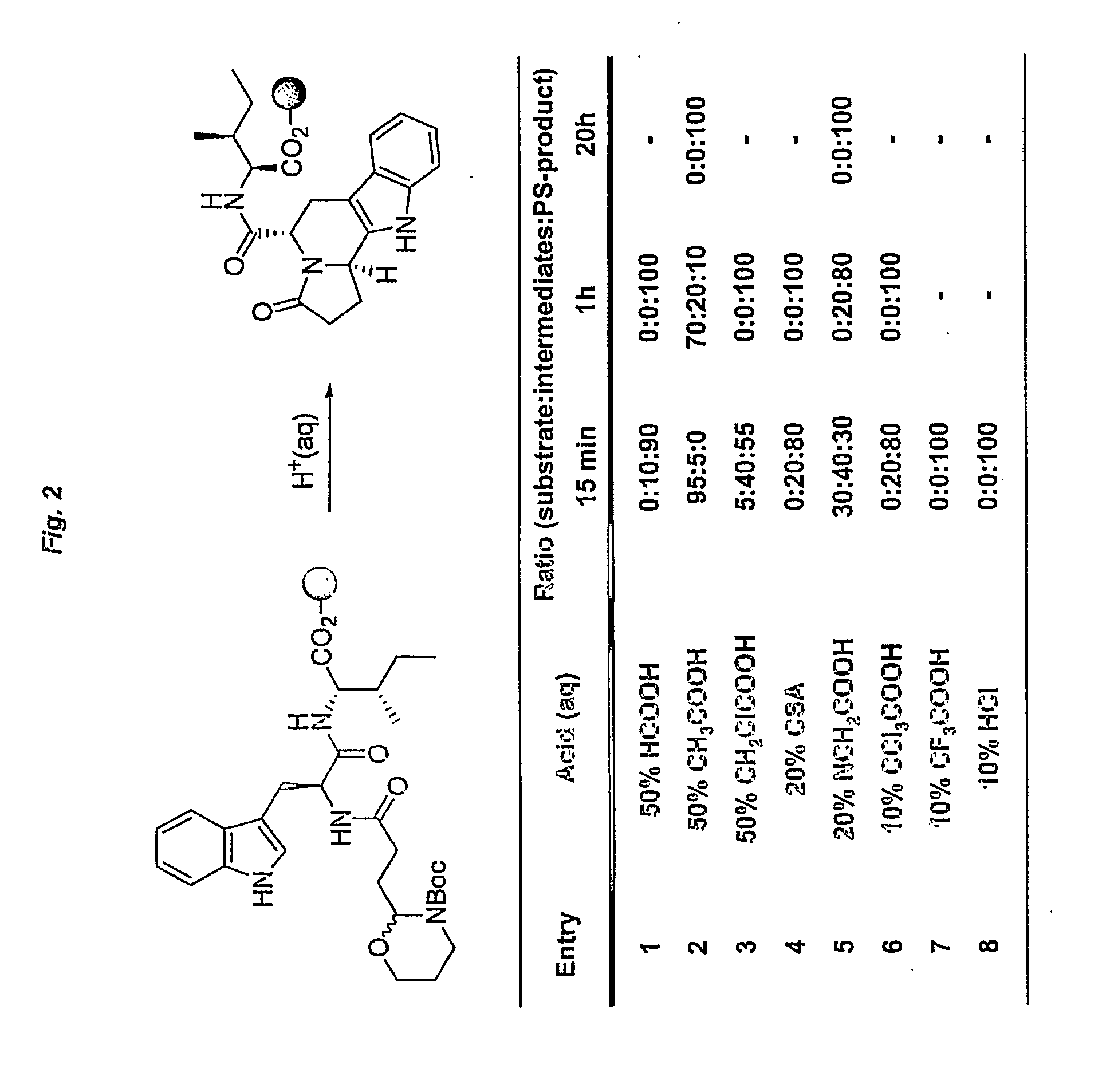
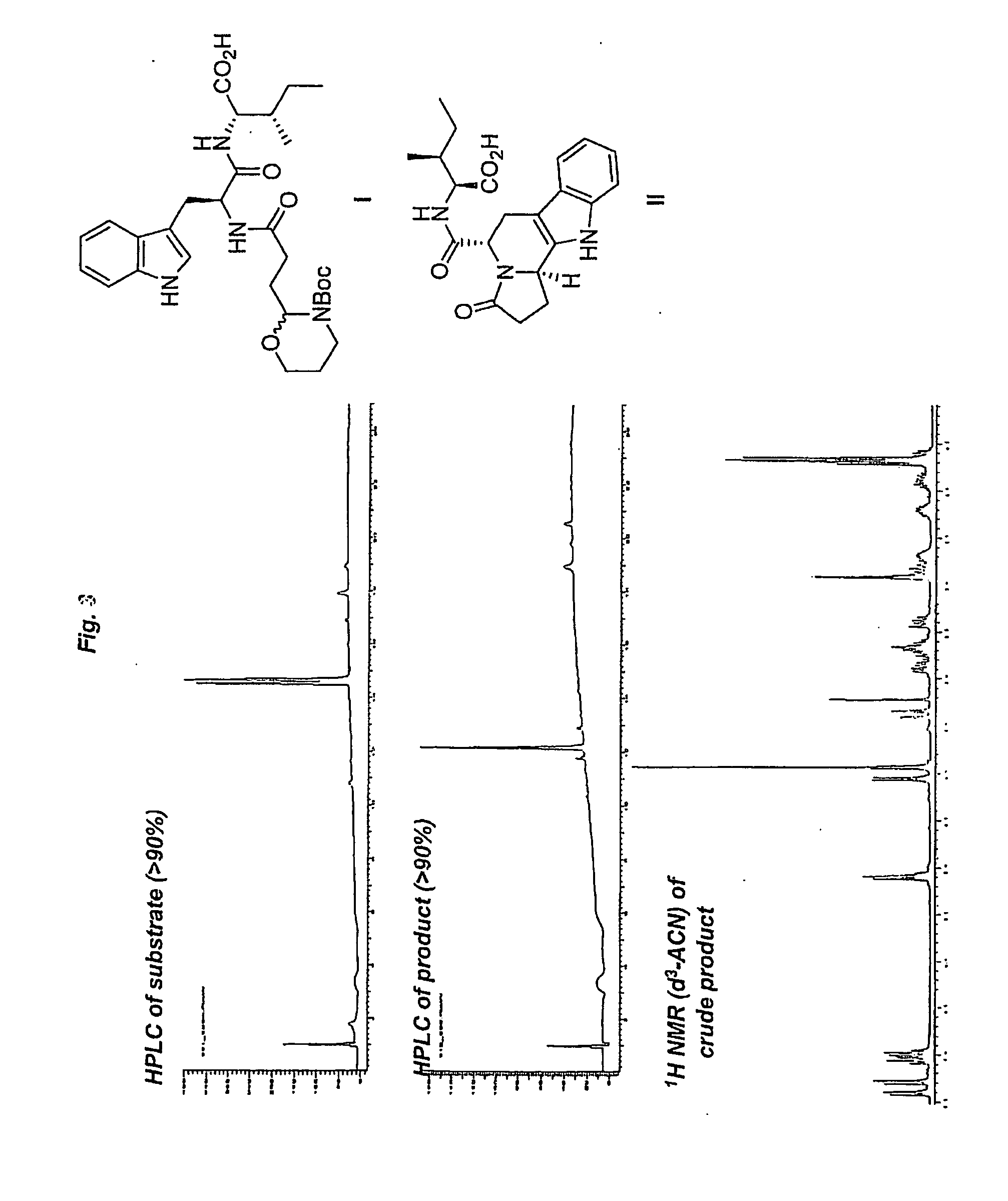
InactiveUS20060252093A1Efficient and rapid accessPromote recoveryPeptide librariesTripeptide ingredientsPictet–Spengler reactionSimple Organic Compounds
The present invention relates to a method of preparing heterocyclic organic com-pounds involving intramolecular formation of an N-acyliminium ion and an in-tramolecular Pictet-Spengler reaction. The invention furthermore discloses precursor molecules useful for the method and methods of preparing these precursor mole-cules. The invention also relates to heterocyclic organic compounds prepared by the methods, libraries of such heterocyclic organic compound and methods of preparing them, as well as to various uses of the heterocyclic organic compounds.
Owner:CARLSBERG BREWERIES AS
Preparation method of fused ring compound containing indole skeleton
The invention discloses a preparation method of a fused ring compound containing an indole skeleton. The preparation method comprises the following steps: carrying out intramolecular Heck reaction under the action of a palladium catalyst, a ligand, an additive and alkali carbonate, and further carrying out cascade reaction by using a sigma-alkyl palladium (II) intermediate generated in situ to obtain the fused ring compound containing the indole skeleton. The one-step method is adopted, construction of a complex fused ring structure is achieved through palladium catalysis, operation is easy, the complex fused ring compound containing the indole skeleton can be obtained without additional treatment, the substrate applicability is wide, the compound can tolerate multiple functional groups, and the product yield is good. An effective means is provided for synthesizing the fused ring compound containing the indole skeleton, and the method has economic practicability and industrial development prospects.
Owner:UNIV OF SCI & TECH OF CHINA
Method for preparing micafungin
InactiveCN103145810AHigh yieldEasy post-processingPeptide preparation methodsBulk chemical productionMicafunginProtecting group
The invention relates to a solid phase-liquid phase full synthetic method for preparing micafungin. The method comprises that Fmoc-P-CTC resin serves as a carrier, a solid phase polypeptide synthetic method is adopted to gradually couple amino acid with an Fmoc protecting group from the C end to the N end, and then the protecting group is deprived through splitting, intramolecular reaction and liquid phase condensation to obtain the micafungin. The method is high in yield, brings convenience to aftertreatment and provides a new thinking way for industrial scale production of the micafungin.
Owner:HYBIO PHARMA
Total synthesis method of optically pure tetrandrine
ActiveCN111518108AFew reaction stepsLow costOrganic chemistry methodsChemical recyclingPtru catalystTetrandrine
The invention discloses a total synthesis method of optically pure tetrandrine, and belongs to the technical field of drug synthesis. The method comprises the following steps: (1) under the action ofa catalyst (1), carrying out intermolecular Ullmann reaction on a compound (1) and a compound (2) under alkaline and high-temperature conditions to synthesize a compound 3; (2) removing a hydroxyl protecting group from the compound (3) under an acidic condition to synthesize a compound (4); (3) carrying out intramolecular Ullmann reaction on the compound (4) under the action of a catalyst (2) under alkaline and high-temperature conditions to synthesize a compound (5), namely the optically pure tetrandrine. A convergent synthesis strategy is adopted, and only three steps are needed from the compound (1) to the synthesis of optically pure tetrandrine so that the reaction steps are greatly reduced, and the time and the material cost are saved; the yield can be as high as 28.7%-38.9%, and theyield is increased by dozens of times; the target product can be obtained on the gram scale, 1.3 g-1. 5 g of final optically pure product is synthesized, and the method has better industrialization potential.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD +1
Method for synthesizing 8th-site substituted-3,4,7,8-tetrahydro-2H-oxocin-2-one octatomic ring
InactiveCN110218203ASimple process conditionsWide applicabilityOrganic chemistryN dimethylformamideSynthesis methods
The invention discloses a method for synthesizing 8th-site substituted-3,4,7,8-tetrahydro-2H-oxocin-2-one octatomic ring. The synthesis method includes the following steps: performing Grignard reaction on an aldehyde or ketone to obtain an allyl alcohol compound; and performing condensation reaction with 2-(tert-butylsulfonyl)acetic acid to obtain an allyl (tert-butylsulfonyl)acetate compound; performing olefin cross-metathesis reaction under the action of a second generation Grubbs catalyst to obtain a methoxycarbonyl (tert-butylsulfonyl)acetate compound; performing intramolecular reaction byusing anhydrous N,N-dimethylformamide as a solvent in the presence of a catalyst palladium acetate and a ligand triphenylphosphine, so as to produce a 3-tert-butylsulfonyl 8th-site substituted-3,4,7,8-tetrahydro-2H-oxocin-2-one octatomic ring lactone compound; and finally removing tert-butylsulfonyl in a reaction solution of sodium amalgam and acetic acid by taking ethanol as a solvent, so as toobtain 8th-site substituted-3,4,7,8-tetrahydro-2H-oxocin-2-one octatomic ring lactone compound. The yield of the method is high, and a solution with a very dilute reaction concentration is not neededin the synthesis of various substituted octatomic ring lactones through intramolecular catalysis by palladium, so the process conditions are simple and universal.
Owner:YANGZHOU UNIV
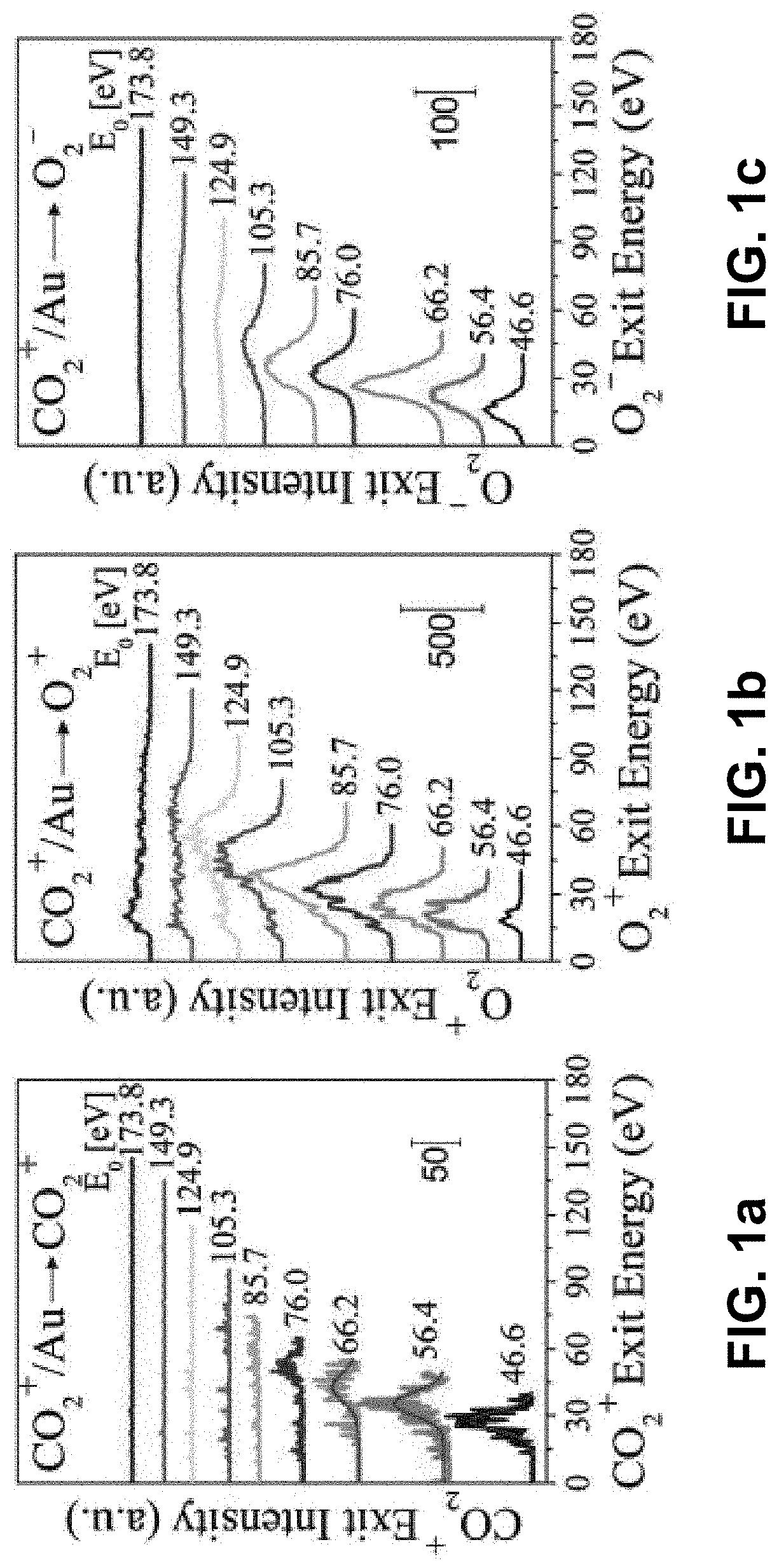
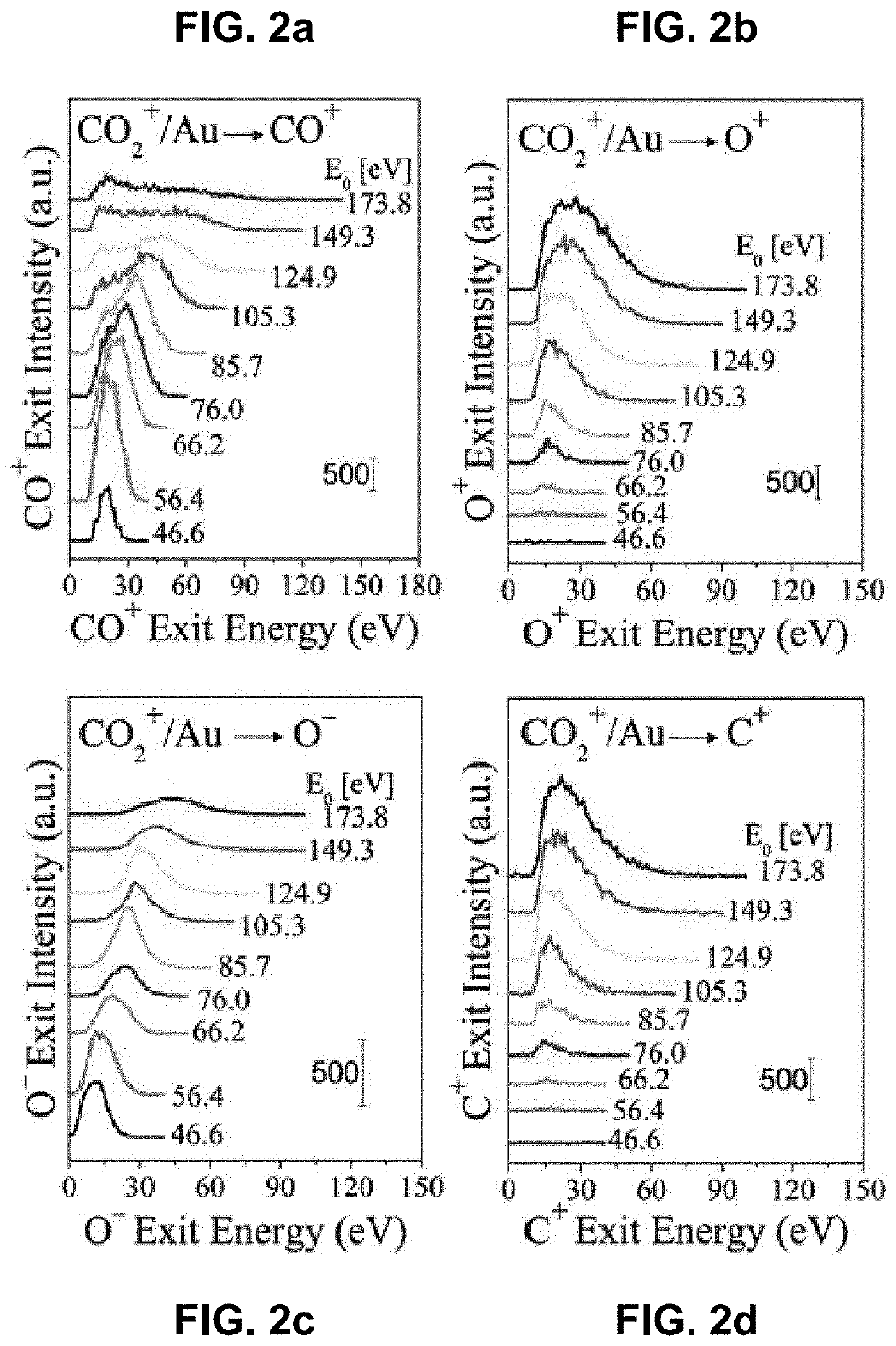
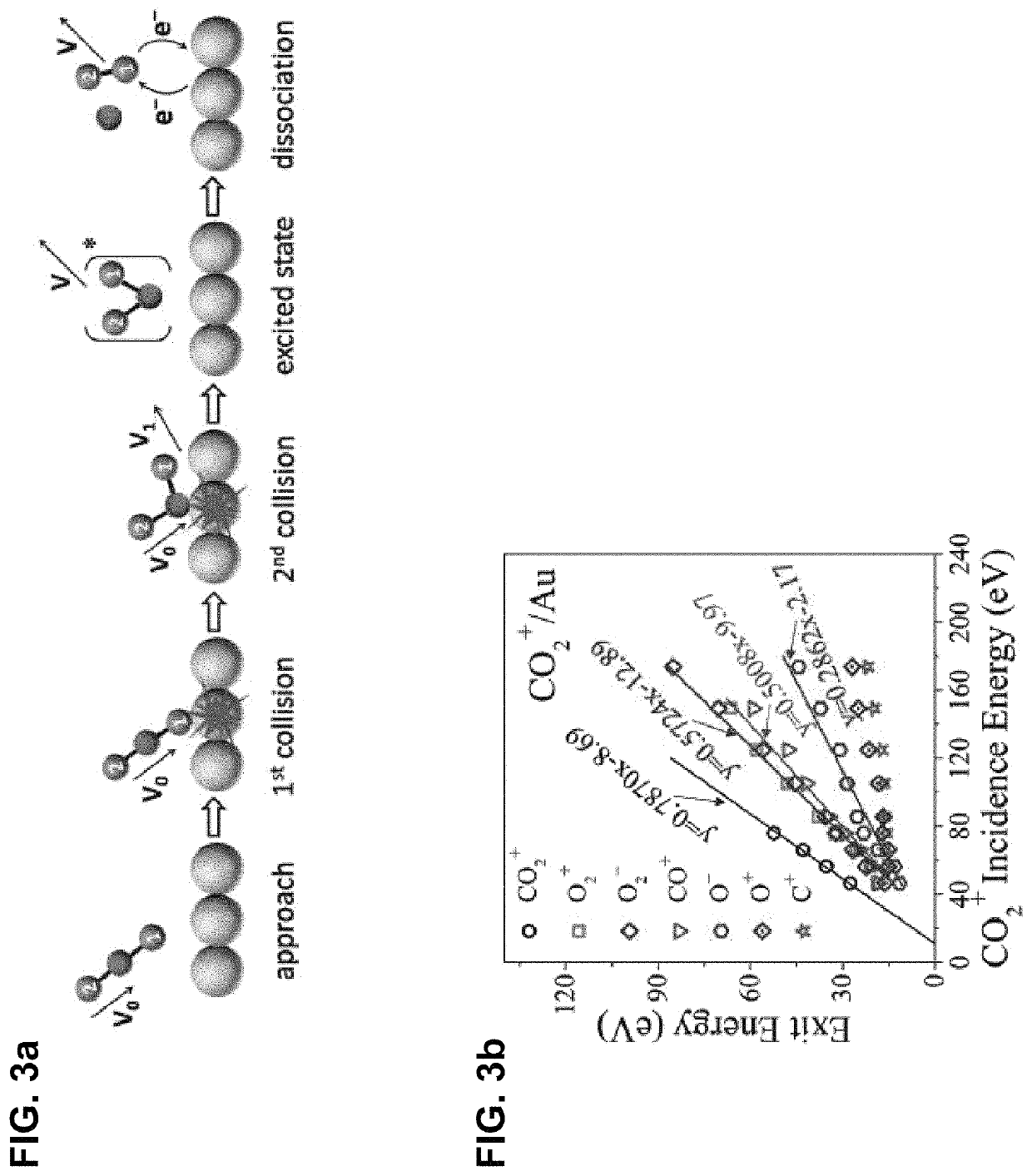
Method for splitting carbon dioxide into molecular oxygen and carbon
Apparatus and methods for facilitating an intramolecular reaction that occurs in single collisions of CO2 molecules (or their derivatives amenable to controllable acceleration, such as CO2+ ions) with a solid surface, such that molecular oxygen (or its relevant analogs, e.g., O2+ and O2− ions) is directly produced are provided. The reaction is driven by kinetic energy and is independent of surface composition and temperature. The methods and apparatus may be used to remove CO2 from Earth's atmosphere, while, in other embodiments, the methods and apparatus may be used to prevent the atmosphere's contamination with CO2 emissions. In yet other embodiments, the methods and apparatus may be used to obtain molecular oxygen in CO2-rich environments, such as to facilitate exploration of extraterrestrial bodies with CO2-rich atmospheres (e.g. Mars).
Owner:CALIFORNIA INST OF TECH
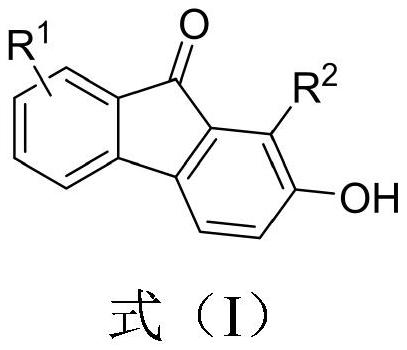
2-hydroxy-9-fluorenone derivative as well as synthesis method and application thereof
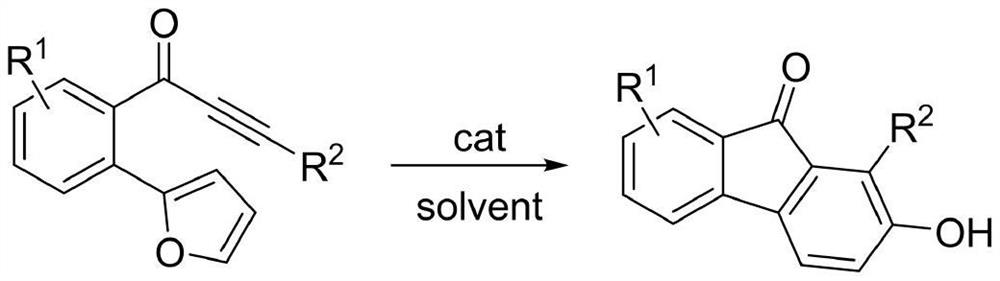
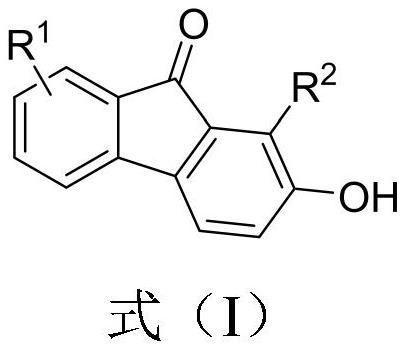
PendingCN113816843AEasy to synthesizeImprove universalityOrganic compound preparationCarbonyl compound preparationFuranPtru catalyst
The invention discloses a 2-hydroxy-9-fluorenone derivative as well as a synthesis method and application thereof. The synthesis of the 2-hydroxy-9-fluorenone derivative shown in the formula (I) is completed through a one-pot method by taking an o-furan aryl alkynone compound as a raw material under the action of a catalyst and utilizing the reaction processes of intramolecular D-A reaction, epoxy ring opening, aromatization and the like of the o-furan aryl alkynone compound. The preparation method provided by the invention has the advantages of simple and easily available raw materials, no metal catalyst, relatively mild reaction conditions, insensitive reaction to air, good substrate universality, simple post-treatment, excellent yield, environmental friendliness and the like. The prepared 2-hydroxy-9-fluorenone derivative can be applied to preparation of anti-virus, anti-tumor, antipyretic and anti-inflammatory drugs, fluorescent probes, sensitized dyes and other functional materials.
Owner:LINYI UNIVERSITY
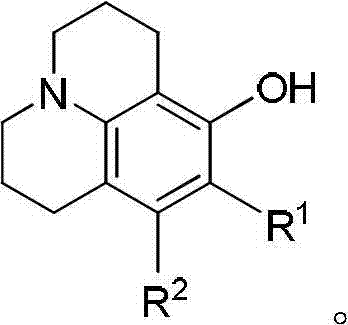
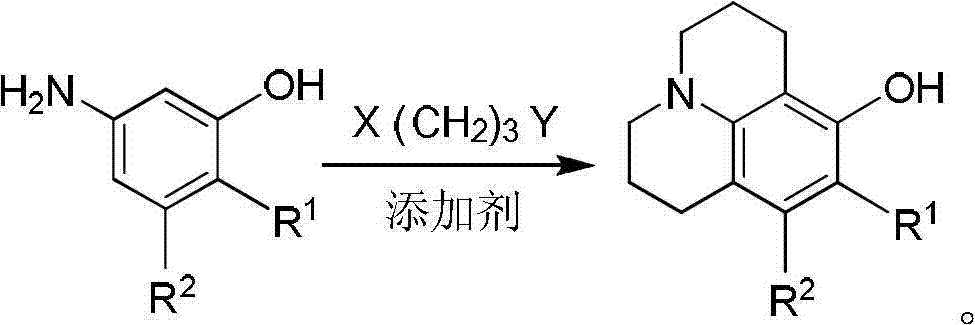
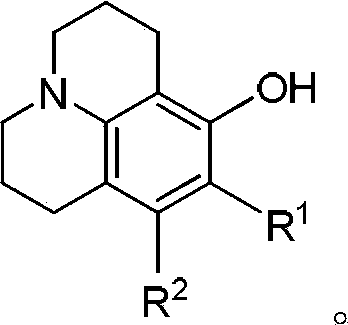
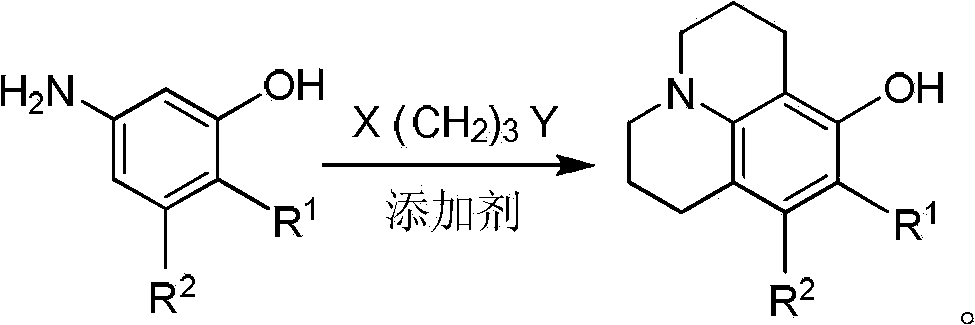
Method for preparing 8-hydroxy julolidine and derivative thereof
The invention discloses a method for preparing 8-hydroxy julolidine and a derivative thereof. The method comprises the following steps of: (1) solving 3-aminophenol compound and X(CH2)3Y in an organic solvent S1, adding alkali B1, performing intramolecular reaction, reacting until the 3-aminophenol compound fully converts into an intermediate, and processing after finishing the reaction to obtain an intermediate; and (2) adding the intermediate obtained in the step (1) and alkali B2 into an organic solvent S2, performing intramolecular reaction, and processing until finishing the reaction to obtain the objective product. The method for preparing the 8-hydroxy julolidine and the derivative thereof is simple in steps; by strictly controlling the reaction stages, occurrence of disubstituted side reaction and oxidation side reaction is reduced, and productivity ratio of the objective product is improved; and high-poisonous and high-polluted substrate or reagent is avoided in the whole reaction process, which is suitable for industrialization.
Owner:ZHEJIANG UNIV
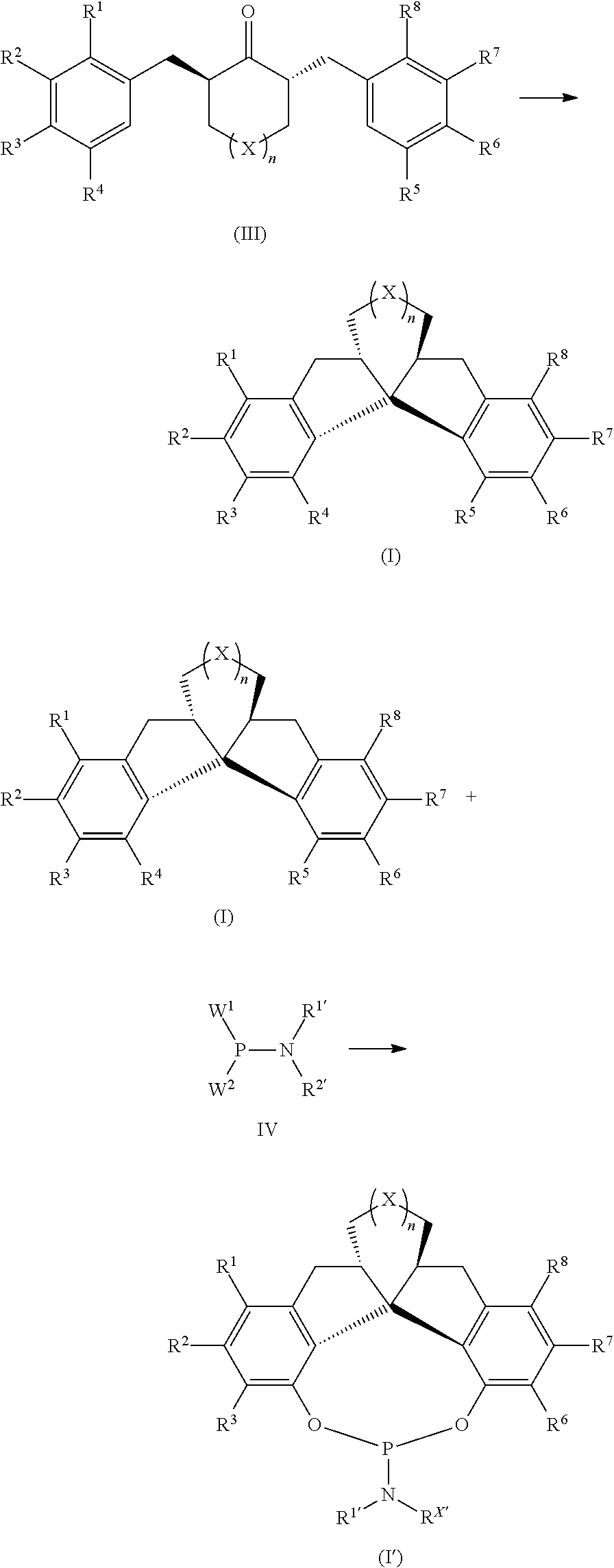
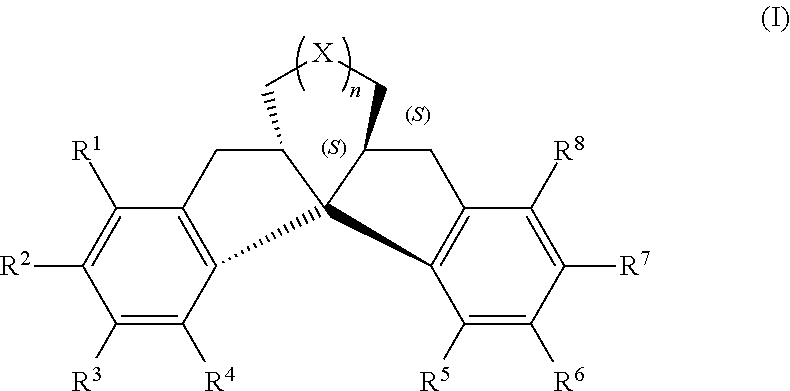
Compound having chiral spirobiindane skeleton and preparation method therefor
ActiveUS11325875B2Poor economicPoor environmentalOrganic reductionOrganic compound preparationPtru catalystHydrogenation reaction
Chiral spirobiindane skeleton compound and preparation method thereof is disclosed in the present invention. The spirobiindane skeleton compound of the present invention having the structure formula of I or I′; the preparation method for synthesizing the spirobiindane skeleton compound of the present invention comprising the following steps: in the presence of solvent and catalysts, the structure formula compound III reacted through intramolecular Friedel-Crafts reaction to obtain the compound of formula I; the catalyst is a Browsteric acidor Lewis acid. The preparation method of chiral fused spirobiindane skeleton compound of the present invention does not need to adopt chiral starting materials or chiral resolving agents, does not require chiral resolving steps, is simple in method, is simple in post-treatment, and is economic and environment friendly. High product yield, high product optical purity and chemical purity. The catalyst for the asymmetric reaction is obtained from the chiral spirobiindane skeleton ligand of the present invention, under the catalytic reagent of transition metal, the catalyzed hydrogenation reaction can arrive at a remarkable catalytic effect with a product yield of >99%, and a product ee value of up to >99%.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD +1
Method for preparing 8-hydroxy julolidine and derivative thereof
The invention discloses a method for preparing 8-hydroxy julolidine and a derivative thereof. The method comprises the following steps of: (1) solving 3-aminophenol compound and X(CH2)3Y in an organic solvent S1, adding alkali B1, performing intramolecular reaction, reacting until the 3-aminophenol compound fully converts into an intermediate, and processing after finishing the reaction to obtain an intermediate; and (2) adding the intermediate obtained in the step (1) and alkali B2 into an organic solvent S2, performing intramolecular reaction, and processing until finishing the reaction to obtain the objective product. The method for preparing the 8-hydroxy julolidine and the derivative thereof is simple in steps; by strictly controlling the reaction stages, occurrence of disubstituted side reaction and oxidation side reaction is reduced, and productivity ratio of the objective product is improved; and high-poisonous and high-polluted substrate or reagent is avoided in the whole reaction process, which is suitable for industrialization.
Owner:ZHEJIANG UNIV
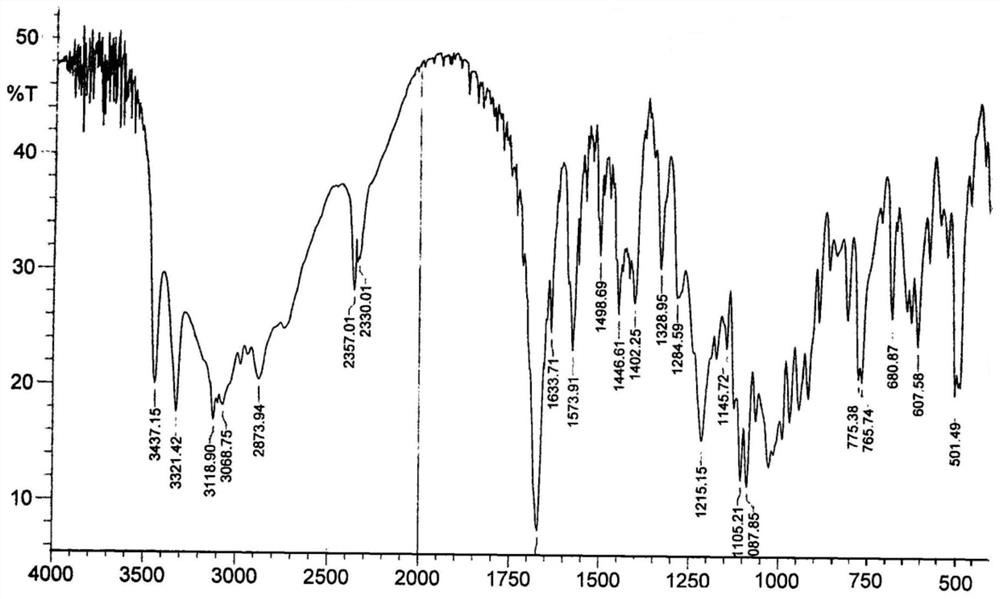
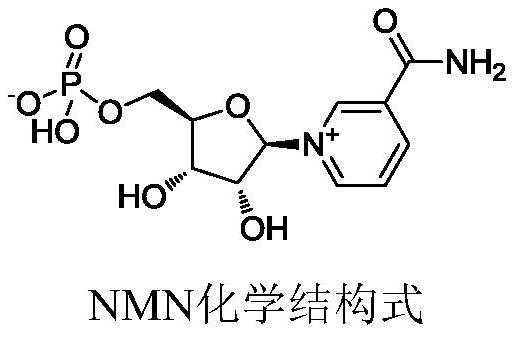
Preparation method of nicotinamide mononucleotide
PendingCN114369128AHigh selectivityIncrease in sizeSugar derivativesSugar derivatives preparationPhosphorylationNucleotide
The invention provides a preparation method of nicotinamide mononucleotide. The preparation method comprises the following steps: carrying out phosphorylation reaction on raw materials containing nicotinamide nucleoside salt, 1, 8-bis (dimethylamino) naphthalene, phosphorus oxychloride and a solvent to obtain a reaction solution containing nicotinamide mononucleotide. On one hand, 1, 8-bis (dimethylamino) naphthalene can inhibit phosphorus oxychloride from continuously attacking 2-position or 3-position hydroxyl; on the other hand, one nitrogen atom of the 1, 8-bis (dimethylamino) is beneficial to removal of hydroxyl hydrogen at the position 2, the other nitrogen atom is beneficial to removal of chlorine in phosphoryl chloride, and removal of hydrogen chloride is facilitated under the synergistic effect of the two nitrogen atoms. Meanwhile, as the distance between two nitrogen atoms in the 1, 8-bis (dimethylamino) naphthalene is short, a transition intermediate formed in the process of removing hydrogen chloride from the 1, 8-bis (dimethylamino) naphthalene is more stable, so that the process of removing hydrogen chloride tends to intramolecular reaction, and the selectivity of phosphorylation reaction is further improved.
Owner:BEIJING HONGHUI MEDITECH CO LTD
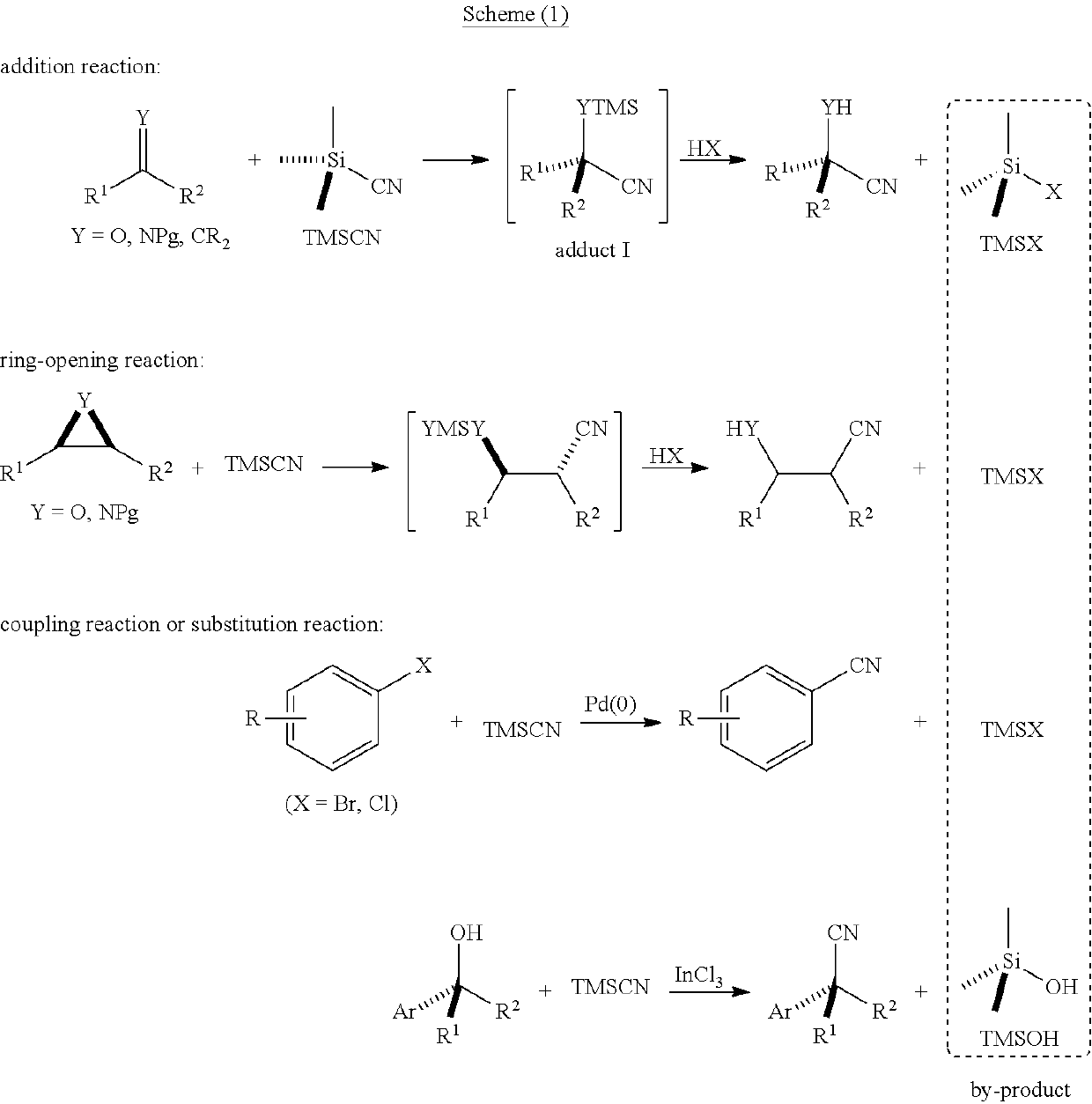
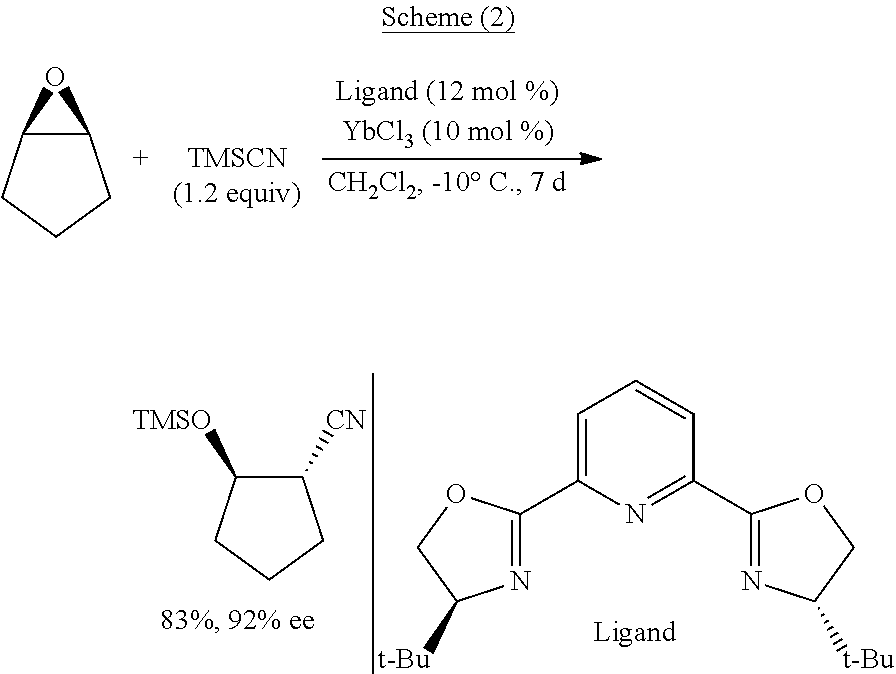
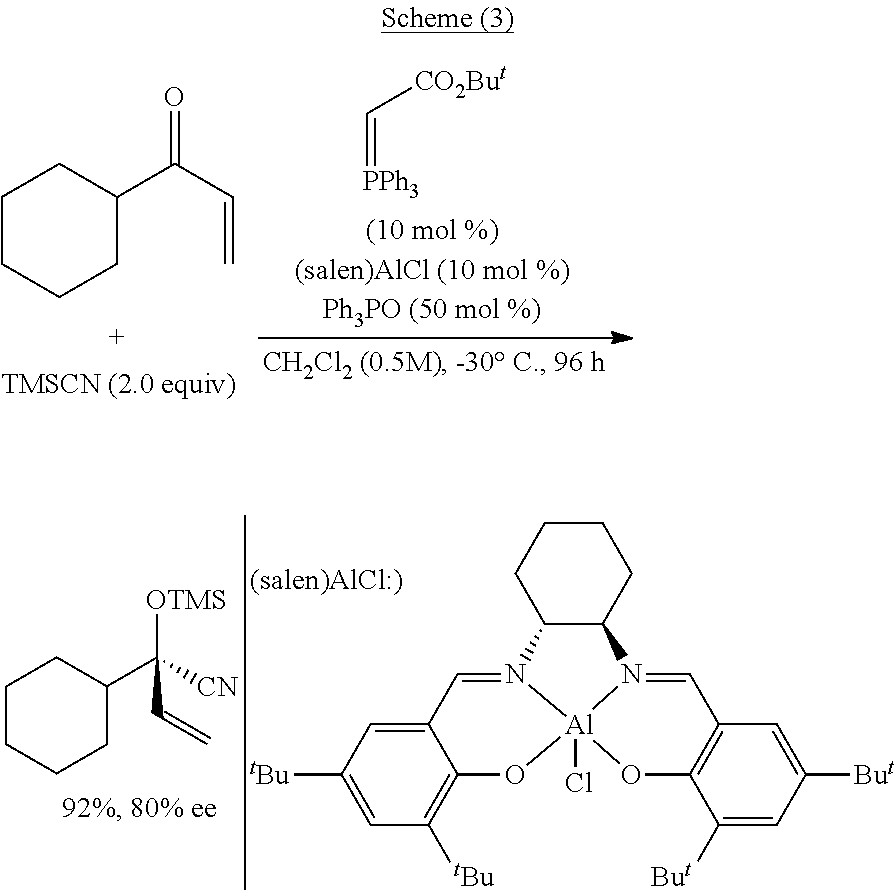
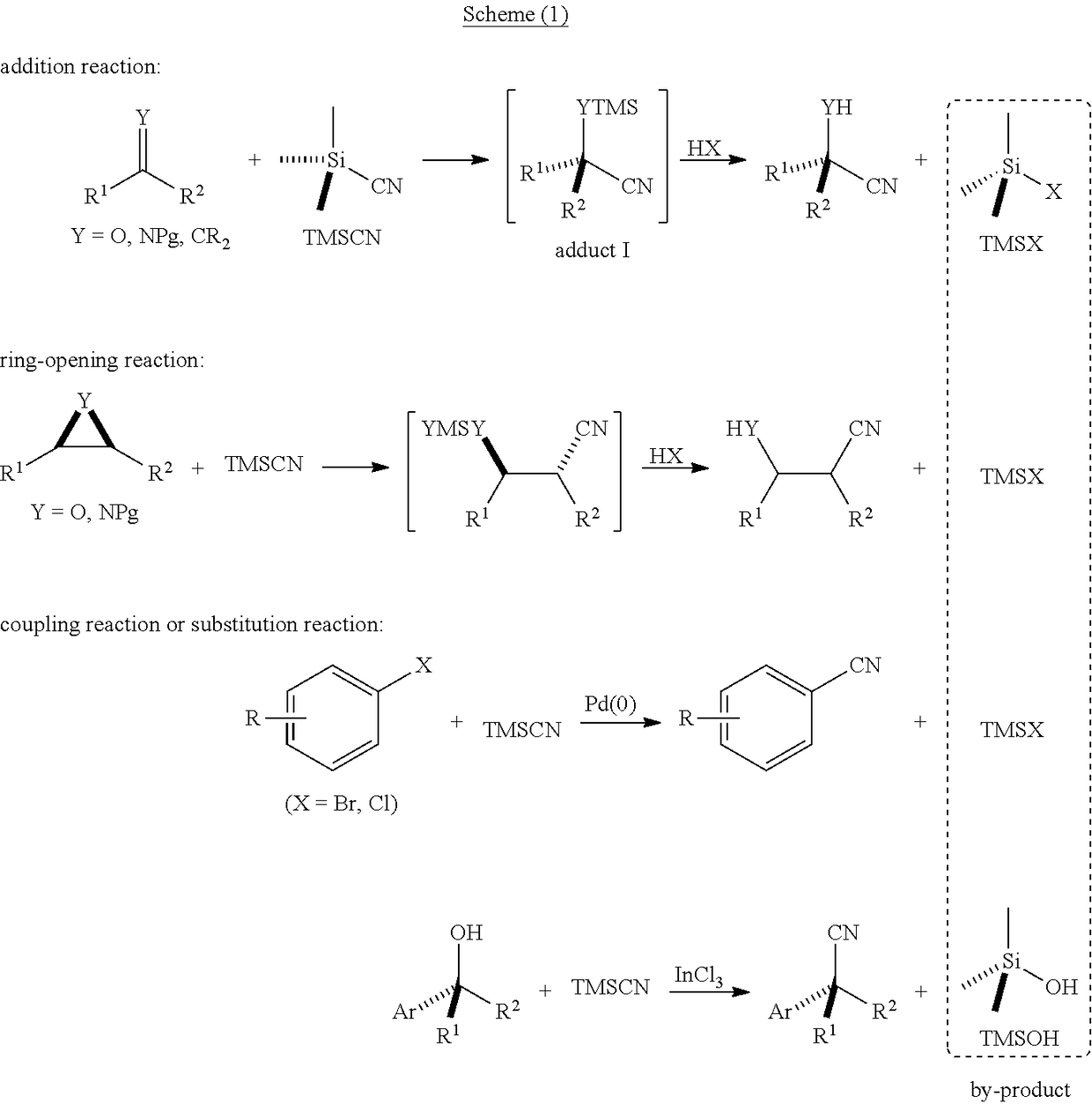
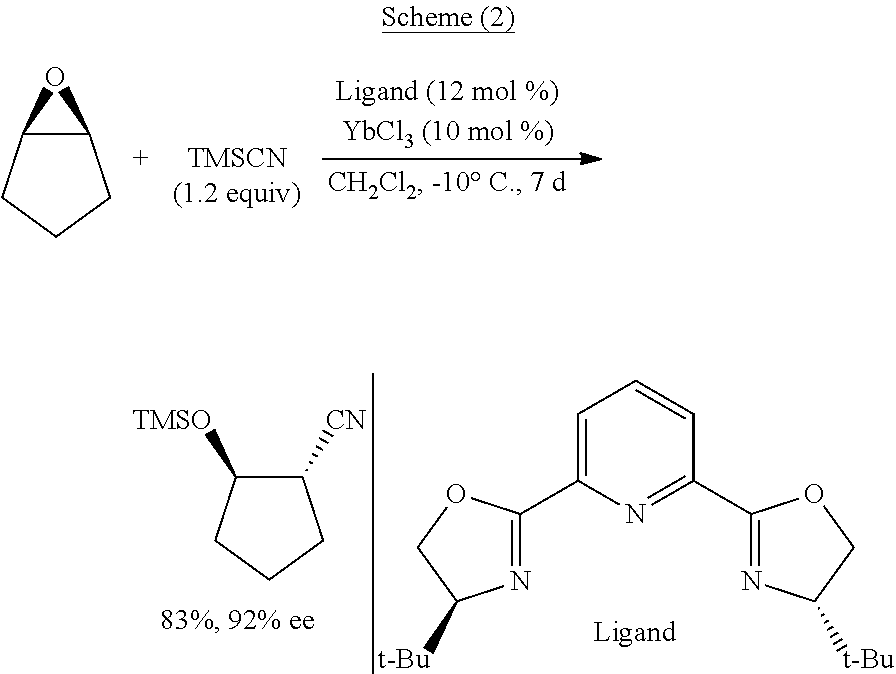
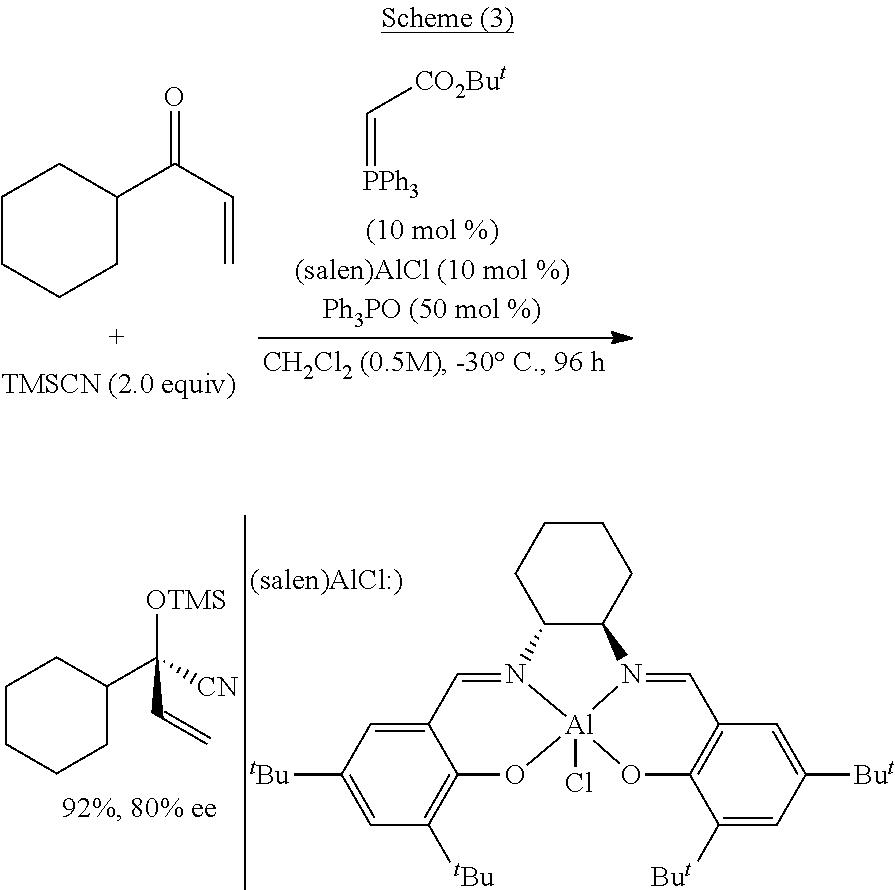
Functionalized cyanosilane and synthesis method and use thereof
ActiveUS10450331B2High yieldAtom utilization is highSilicon organic compoundsComponent separationSilyleneSynthesis methods
The present teachings relate to a functionalized silyl cyanide and synthetic methods thereof. As an example, the method may include adding a raw material silane and a cyanide source MCN in an organic solvent to produce the functionalized silyl cyanide in the absence of catalyst or in the presence of a metal salt catalyst. The functionalized silyl cyanide may be used in the reactions that classic TMSCN participates in, to synthesize important intermediates (e.g., cyanohydrin, amino alcohols and α-amino nitrile compounds), with improved reactivity and selectivity. The cyanosilyl ether resulted from the nucleophilic addition of functionalized silyl cyanide to aldehyde or ketone may undergo intramolecular reaction under appropriate conditions to transfer the functional groups on silicon onto the other parts of the product linked to silicon. Such a functional group transfer process may increase the synthesis efficiency and atom economy, as well as afford products unobtainable using traditional TMSCN.
Owner:EAST CHINA NORMAL UNIV
Functionalized cyanosilane and synthesis method and use thereof
ActiveUS20180141964A1High yieldAtom utilization is highSilicon organic compoundsOrganic compound preparationSilyleneSilanes
The present teachings relate to a functionalized silyl cyanide and synthetic methods thereof. As an example, the method may include adding a raw material silane and a cyanide source MCN in an organic solvent to produce the functionalized silyl cyanide in the absence of catalyst or in the presence of a metal salt catalyst. The functionalized silyl cyanide may be used in the reactions that classic TMSCN participates in, to synthesize important intermediates (e.g., cyanohydrin, amino alcohols and α-amino nitrile compounds), with improved reactivity and selectivity. The cyanosilyl ether resulted from the nucleophilic addition of functionalized silyl cyanide to aldehyde or ketone may undergo intramolecular reaction under appropriate conditions to transfer the functional groups on silicon onto the other parts of the product linked to silicon. Such a functional group transfer process may increase the synthesis efficiency and atom economy, as well as afford products unobtainable using traditional TMSCN.
Owner:EAST CHINA NORMAL UNIV
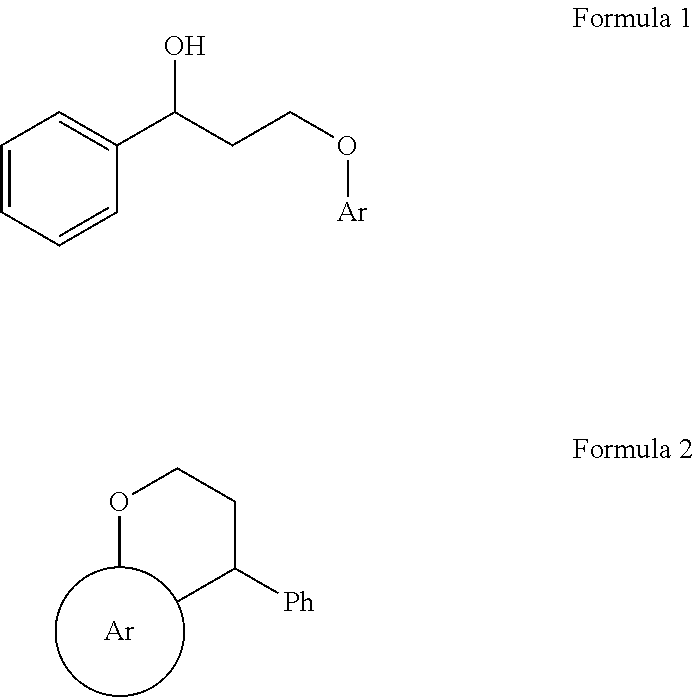
Process for the production of 4-substituted chromanes via gold catalysis
InactiveUS9102646B2High yieldEfficient synthesisOrganic chemistryChemical recyclingArylCombinatorial chemistry
Disclosed herein is single step process for the synthesis of 4-aryl substituted chromanes of compound of formula 2 comprising subjecting 3-aryloxy-1-phenylpropan-1-ol of formula 1 to (III) chloride-catalyzed intramolecular Friedel-Crafts reaction to obtain 4-aryl substituted chromanes. The invention further discloses novel 4-substituted Chromane compounds.
Owner:COUNCIL OF SCI & IND RES
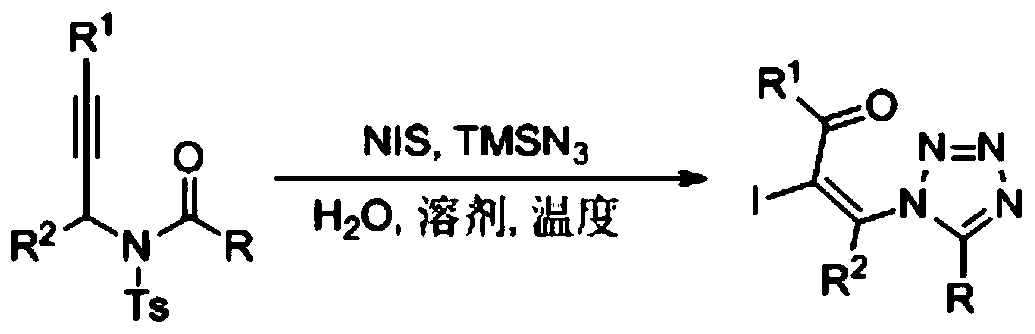
A kind of preparation method of 1,5-disubstituted tetrazole compound
The invention relates to a preparation method of a 1,5-disubstituted tetrazole compound. The specific method is to use propynylamide which is simply prepared as a raw material, and under the conditions of N-iodosuccinimide (NIS), azidotrimethylsilane (TMSN3) and water, intramolecular oxygen atom transfer occurs The reaction yields 1,5-disubstituted tetrazole compounds. Propigylamide can be easily prepared from cheap and easy-to-obtain starting materials, and TMSN3 is used as a nitrogen source, and an intramolecular oxygen atom transfer reaction occurs in one step to obtain 1,5-disubstituted tetrazole. A metal catalyst is required.
Owner:中科榆林能源技术运营有限责任公司
Preparation method of phenyl sulfide compound
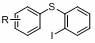
The invention discloses a preparation method of a phenyl sulfide compound, which comprises the following steps: by taking thiophenol and o-diiodobenzene as substrates, reacting in a solvent in the presence of a metal hydride to obtain iodine-containing phenyl sulfide; the iodine-containing phenyl sulfide can be subjected to intramolecular reaction to obtain a phenyl sulfide compound; or the iodine-containing phenyl sulfide can react with other raw materials to obtain the phenyl sulfide compound. The ortho-iodine compound prepared by the invention has great application value, can be further widely converted, can be subjected to coupling reaction with phenylboronic acid, thiophenol, phenylacetylene and the like under the catalysis of Pd to prepare various 2-substituted thiophenol, and has important significance in material science and pharmaceutical synthesis.
Owner:SUZHOU UNIV
Simple synthesis method of 1-alkyl-3-aryl substituted indolizine compound
The invention discloses a simple synthesis method of a 1-alkyl-3-aryl substituted indolizine compound, and belongs to the field of organic synthesis. According to the method, (E)-4-aryl-2-pyridyl-3-alkenyl-2-alcohol compounds 1 are used as raw materials and react under acid catalysis to obtain 1-alkyl-3-aryl substituted indolizine compounds 2, wherein the acid catalysis comprises two methods of catalysis in an inorganic acid aqueous solution and catalysis in an organic acid / organic solvent. The two methods provided by the invention are both completed through an acid catalytic dehydration intramolecular reaction, have different catalytic effects on substrates containing different substituent groups, and can complement each other's advantages; and meanwhile, the method has the advantages of simple and easily available raw materials, simple operation, wide substrate application range and the like.
Owner:HENAN NORMAL UNIV
Method of Preparing a Ring Compound Having Two Adjacent Chiral Centers
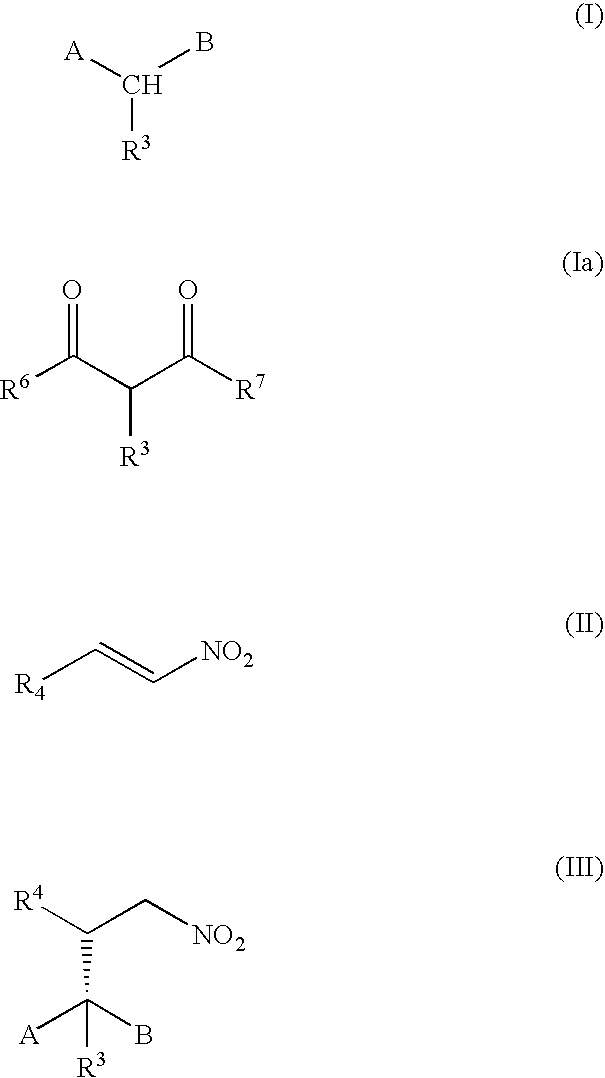
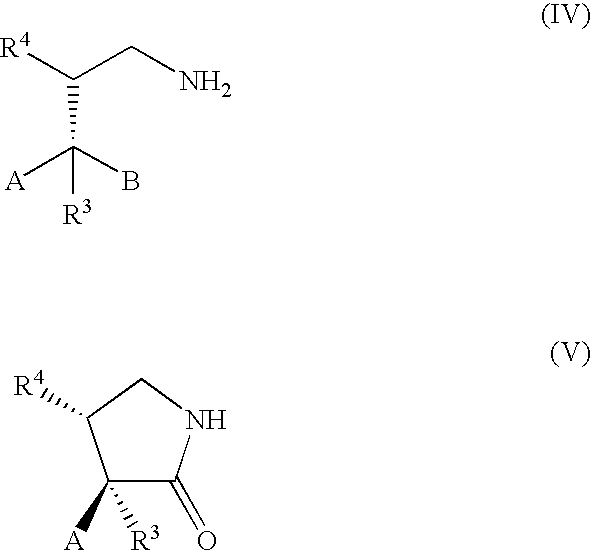
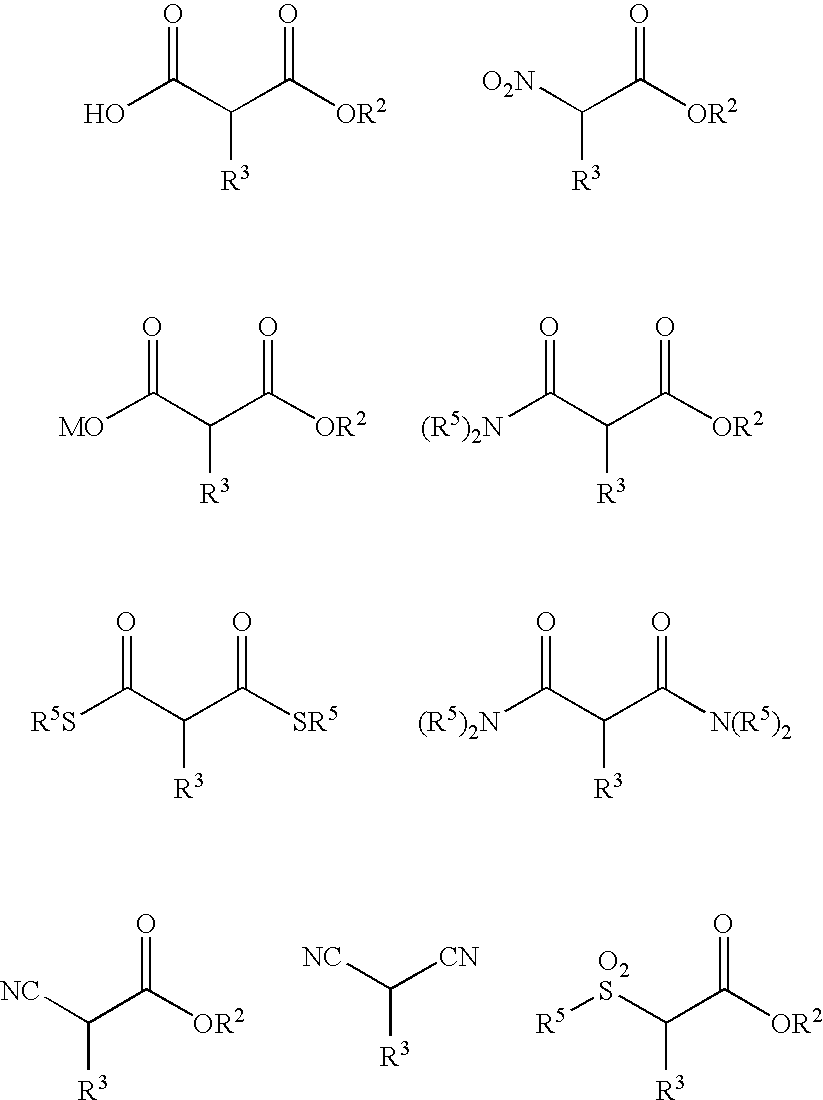
InactiveUS20070276145A1High yieldEfficient synthesisOrganic chemistryOrganic compound preparationNitroalkeneIntramolecular reaction
A method of synthesizing a chiral compound having a quaternary carbon atom bearing diastereotopic groups from (a) a nitroolefin and (b) an α-substituted β-dicarbonyl or an equivalent compound having an acidic C—H moiety compound is disclosed. A subsequent intramolecular reaction between one of the substituents comprising the stereogenic carbon atom and one of the diastereotopic groups comprising the quaternary carbon atom creates a new compound having two contiguous stereogenic centers, one of which is quaternary, with control over the relative stereochemistry.
Owner:ICOS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com