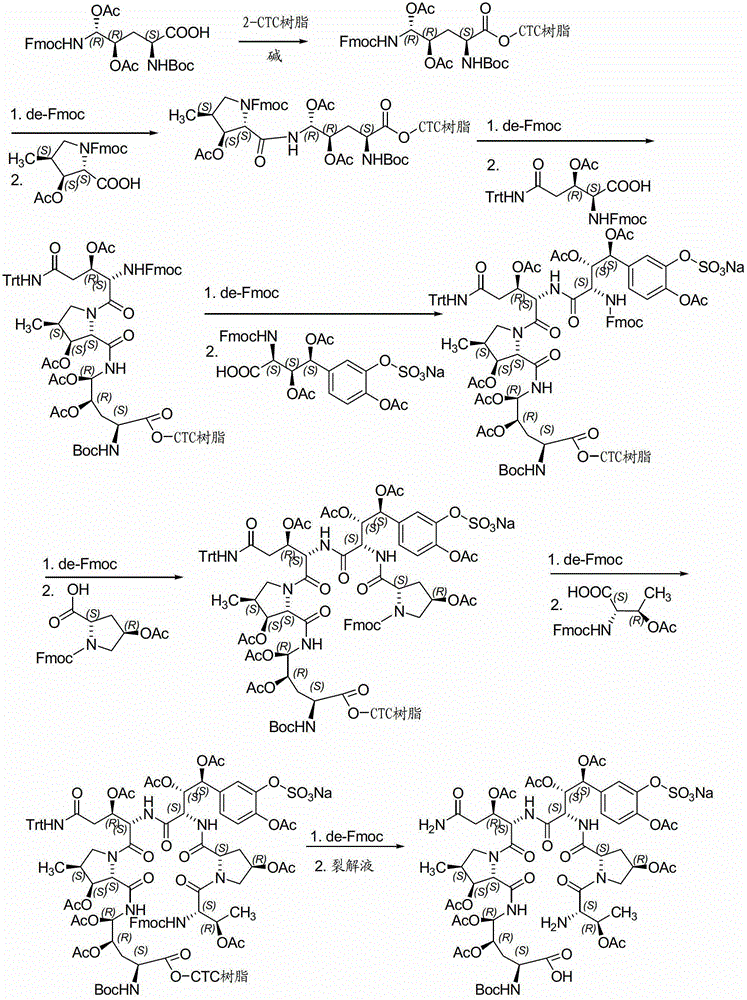
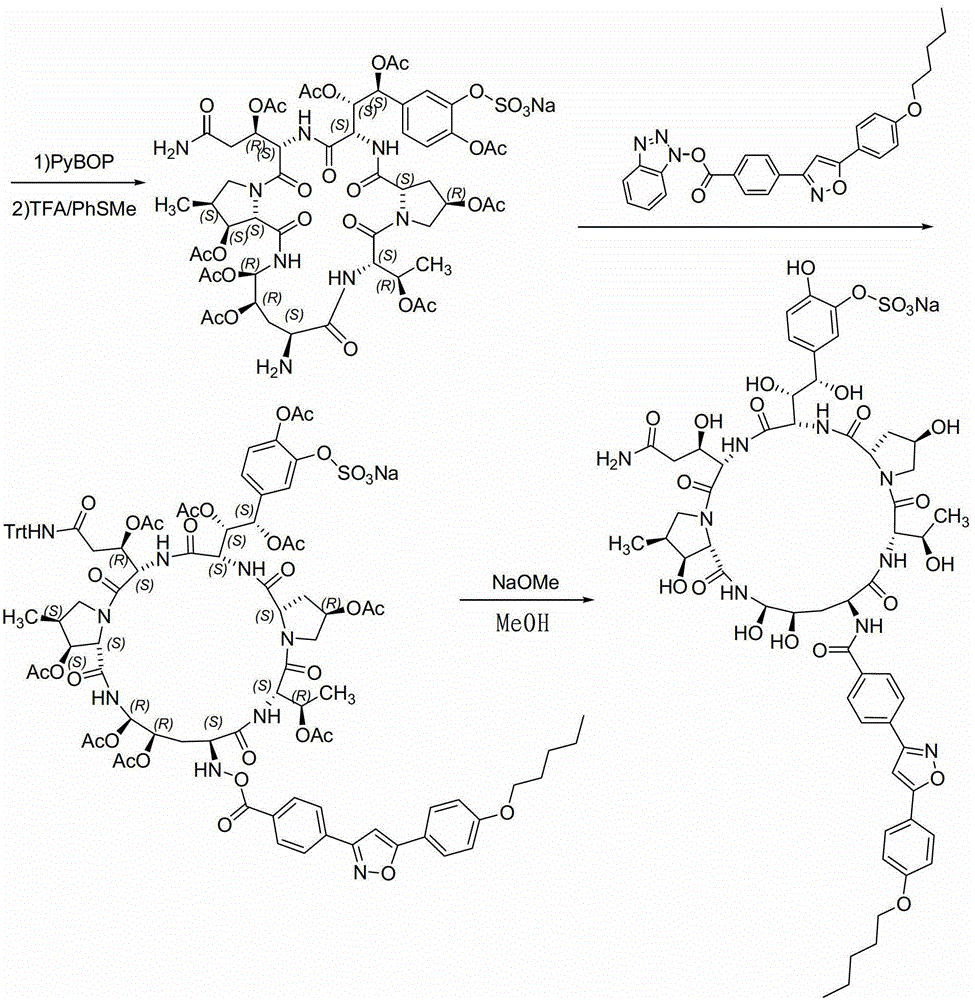
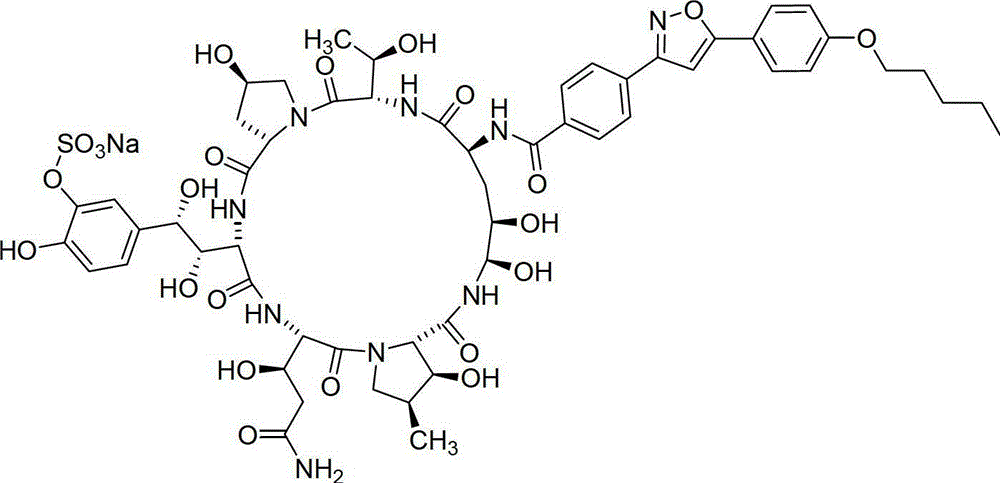
A kind of method for preparing micafungin
A technology of micafungin and protecting group, which is applied in the field of preparing micafungin, can solve problems such as needs, and achieve the effects of high yield and convenient post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Fmoc-P-OH unnatural amino acid is synthesized through the following three steps:
[0043] (1) Synthesis of ortho-dihydroxyl groups by Sharpless asymmetric dihydroxylation reaction. 70g (217mmol) K 3 Fe(CN) 6 , 29.3g (217mmol) K 2 CO 3 , 53.2 mg (0.14 mmol) K 2 OSo 2 (OH) 4 And 0.56g (0.7mmol) hydroquinidine 1,4-(2,3-naphthyridine) diether ((DHQD) 2 -PHAL) was dissolved in 350ml tert-butanol and 350ml water, and stirred at room temperature for 10 minutes. Add 6.7g (70mmol) amine mesylate, at 0°C, Boc-4-ene-L-ornithinolactam (JournalofOrganometallicChemistry691 (2006), 5487-5496, experimental part) prepared according to the method of known literature 4.3.3, Compound 24) 14.9g (70mmol) was added to the system, and kept at 0°C for 40h. Subsequently, 105 g (833 mmol) of sodium sulfite was added, the reaction was naturally raised to room temperature and stirred for 1 hour, and 700 ml of ethyl acetate was added. The aqueous layer was extracted with ethyl a...
Embodiment 2
[0046] Embodiment 2: the synthesis of Fmoc-P-CTC resin
[0047] Weigh 20.0 g of 2-CTC resin with a substitution degree of 0.7 mmol / g, add it to a solid-phase reaction column, and wash it twice with DMF. After swelling the resin with DMF for 30 minutes, take 24.0g of Fmoc-P-OH and dissolve it in DMF, add 14.6mL of DIPEA in an ice-water bath to activate for 3 minutes, then add it to the above-mentioned reaction column filled with resin, react for 2 hours, add 20mL of anhydrous Methanol blocked for 1 hour. Wash 3 times with DMF and 3 times with DCM, seal with anhydrous methanol for 30 minutes, shrink and dry with methanol to obtain Fmoc-P-CTC resin, the detection degree of substitution is 0.56mmol / g.
Embodiment 3
[0048] Example 3: Synthesis of (4S)-N-Fmoc-4-acetoxy-4-(3'-sodium sulfonate-4'-acetoxy)phenyl-L-threonine:
[0049] Dissolve 50 g (185 mmol) of 3'-acetoxy-4'-benzyloxy-benzaldehyde (Org. Biomol. Chem., 2010, 8, 5199–5211 experimental part compound 29) in 250 ml of freshly distilled DCM, add 101.4 g (185 mmol) of phosphine ylides were reacted with stirring at room temperature for 4 hours, and the reaction liquid was purified by silica gel flash column chromatography to obtain 80.1 g of white solid. Dissolve this solid in 300ml of freshly distilled DCM, add a solution of 15g (277mmol) sodium methoxide in 2300ml of anhydrous methanol, stir and react at room temperature for 30 minutes, add 1500ml of 1mol / L hydrochloric acid solution to acidify, spin off the organic solvent, and use the remaining liquid DCM extraction (3 x 600ml). The organic phases were combined, washed twice with saturated brine, dried over anhydrous sodium sulfate for 2 h, and then concentrated under reduced pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com