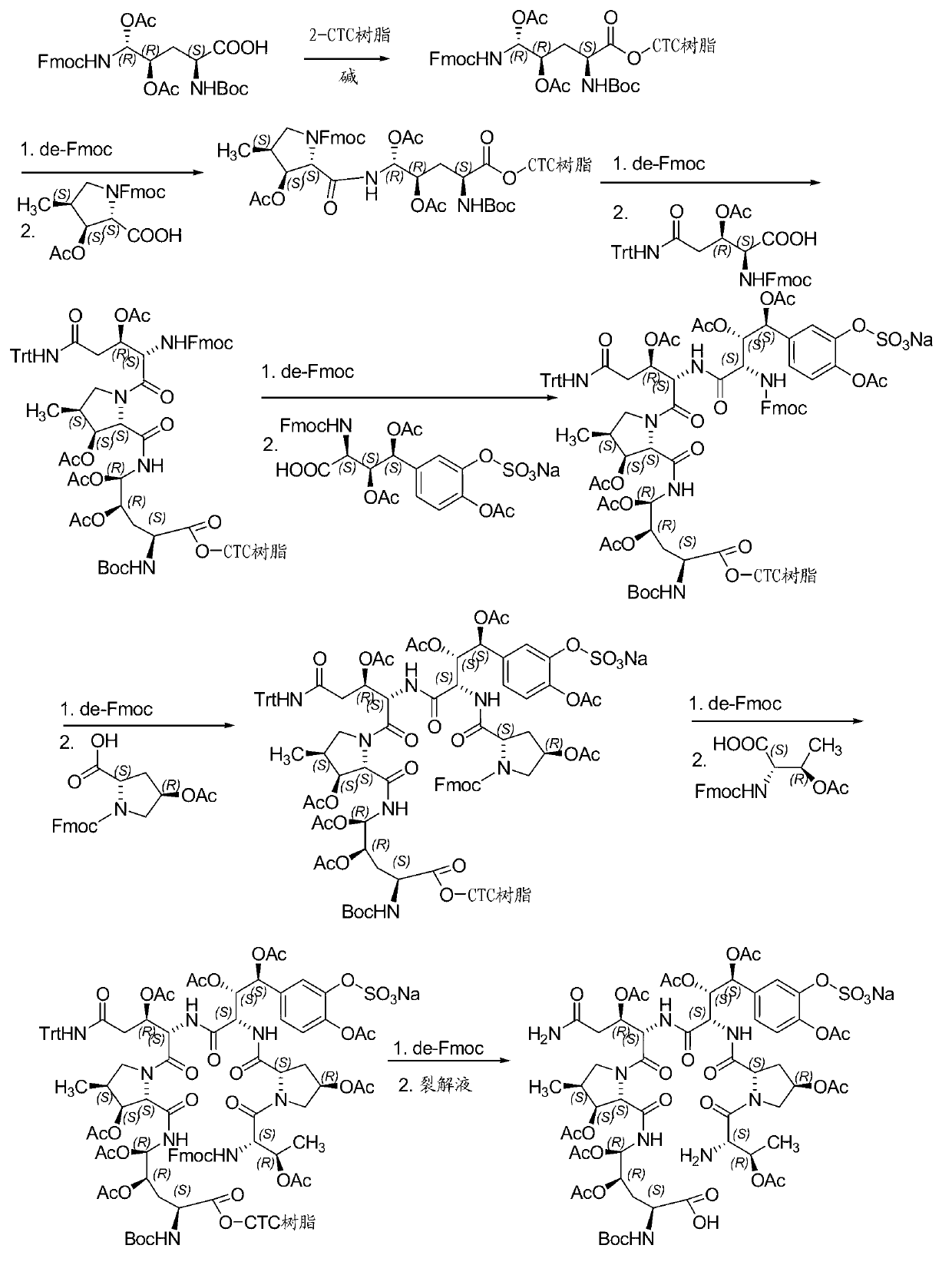
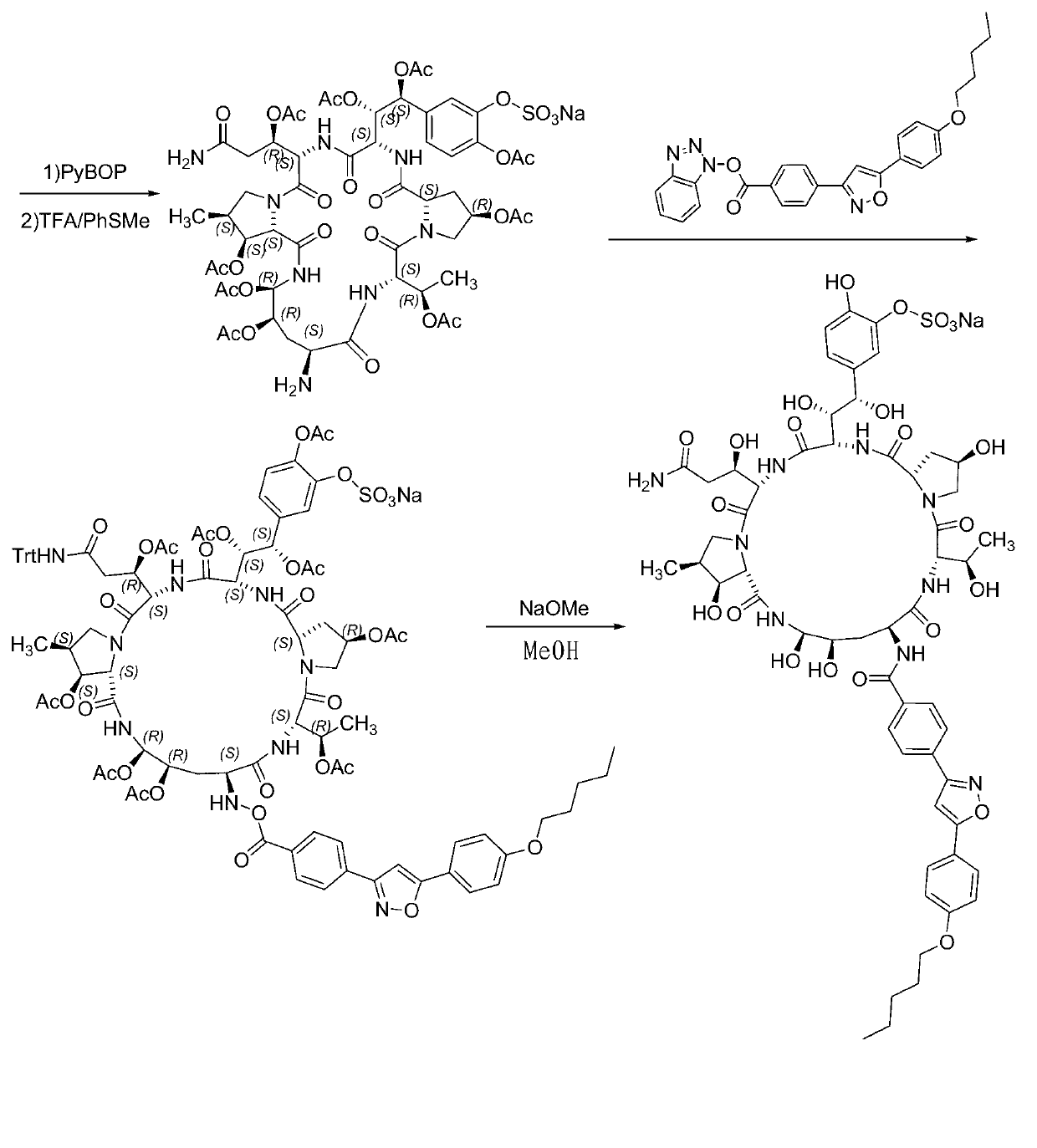
Method for preparing micafungin
A technology of micafungin and protecting group, which is applied in the field of preparing micafungin, can solve problems such as needs, and achieve the effects of high yield and convenient post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
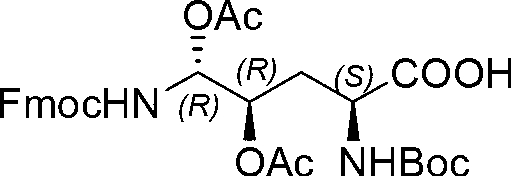
[0042] Example 1: Fmoc-P-OH unnatural amino acid was synthesized by the following three steps:
[0043] (1) Synthesis of ortho-dihydroxyl groups by Sharpless asymmetric dihydroxylation. 70g (217mmol) K 3 Fe(CN) 6 , 29.3g(217mmol)K 2 CO 3 , 53.2 mg (0.14 mmol) K 2 OsO 2 (OH) 4 and 0.56g (0.7mmol) hydroquinidine 1,4-(2,3-naphthalene) diether ((DHQD) 2 -PHAL) was dissolved in 350 ml of t-butanol and 350 ml of water and stirred at room temperature for 10 minutes. Add 6.7 g (70 mmol) of amine methanesulfonate, and at 0 °C, Boc-4-ene-L-ornithine lactam prepared according to the method of the known literature (Journal of Organometallic Chemistry 691 (2006), 5487-5496 , experimental part 4.3.3, compound 24) 14.9 g (70 mmol) was added to the system, and the reaction was kept at 0° C. for 40 h. Subsequently, 105 g (833 mmol) of sodium sulfite was added, and the reaction was stirred at room temperature for 1 hour, and 700 ml of ethyl acetate was added. The aqueous layer was ext...
Embodiment 2
[0046] Example 2: Synthesis of Fmoc-P-CTC resin
[0047] 20.0 g of 2-CTC resin with a substitution degree of 0.7 mmol / g was weighed, added to the solid-phase reaction column, and washed twice with DMF. After the resin was swollen with DMF for 30 minutes, 24.0 g of Fmoc-P-OH was dissolved in DMF, 14.6 mL of DIPEA was added under an ice-water bath for activation for 3 minutes, and then added to the above-mentioned reaction column containing the resin. After 2 hours of reaction, 20 mL of DIPEA was added. Water methanol blocked for 1 hour. Washed 3 times with DMF, washed 3 times with DCM, blocked with anhydrous methanol for 30 minutes, shrunk with methanol and drained to obtain Fmoc-P-CTC resin, and the detected substitution degree was 0.56 mmol / g.
Embodiment 3
[0048] Example 3: Synthesis of (4S)-N-Fmoc-4-acetoxy-4-(3'-sodium sulfonate-4'-acetoxy)phenyl-L-threonine:
[0049] 50 g (185 mmol) of 3'-acetoxy-4'-benzyloxy-benzaldehyde (Org. Biomol. Chem., 2010, 8, 5199-5211 experimental part compound 29) was dissolved in 250 ml of freshly distilled DCM, added 101.4 g (185 mmol) of phosphine ylide was stirred at room temperature for 4 hours, and the reaction solution was purified by silica gel flash column chromatography to obtain 80.1 g of a white solid. This solid was dissolved in 300ml of freshly distilled DCM, a solution of 15g (277mmol) of sodium methoxide in 2300ml of anhydrous methanol was added, and after stirring at room temperature for 30 minutes, 1500ml of 1mol / L hydrochloric acid solution was added to acidify, and the organic solvent was removed, and the remaining liquid was Extract with DCM (3x600ml). The organic phases were combined, washed twice with saturated brine, dried over anhydrous sodium sulfate for 2 h, and concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com