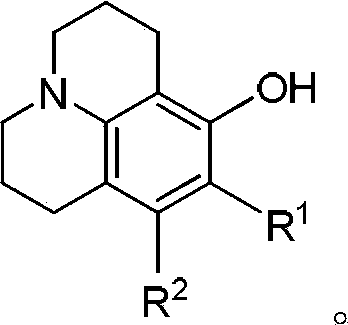
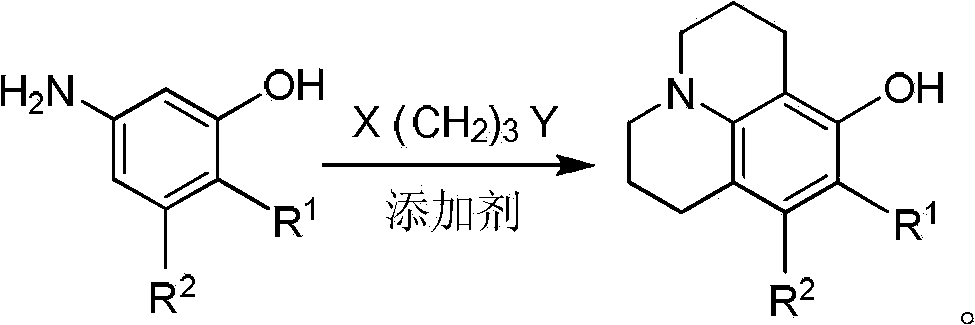
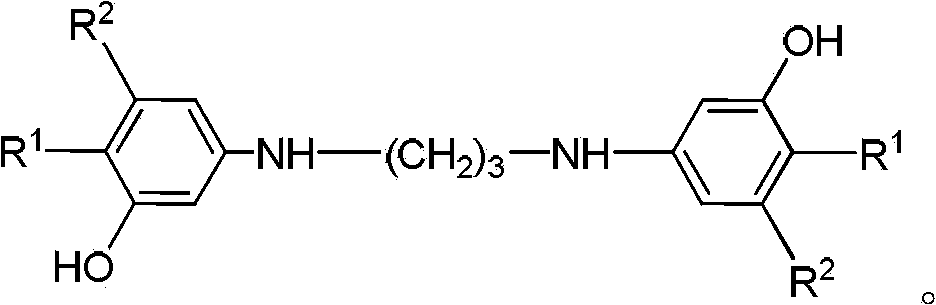Method for preparing 8-hydroxy julolidine and derivative thereof
A technology of derivatives and compounds, which is applied in the field of preparation of 8-hydroxyjulonidine and derivatives thereof, can solve the problems of low total yield, environmental harm and high production cost, and achieves reduction of side reactions of disubstituted propane and reduction of oxidation Side effects, simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 40mmol of 3-aminophenol and 160mmol of 1-bromo-3-chloropropane were dissolved in 30ml of N,N-dimethylformamide, 80mmol of sodium bicarbonate was added, the reactant was protected by nitrogen, and stirred at room temperature under nitrogen Reacted for 12 hours, TLC detected that the 3-aminophenol had reacted completely, stopped the reaction, poured the reaction system into 150ml of water, extracted the generated intermediate with ethyl acetate, dried over anhydrous magnesium sulfate, removed the solvent, and obtained Intermediates were used directly in subsequent reactions.
[0035] Dissolve the intermediate obtained above into 30ml of N,N-dimethylformamide, add 80mmol of sodium bicarbonate, protect the reaction process with nitrogen, stir at room temperature under nitrogen protection and continue the reaction for 12 hours, TLC detection until the reaction is complete, the reaction After the end, the reaction system liquid was added to 100ml of water, and the target prod...
Embodiment 2
[0041] 40mmol of 3-aminophenol and 240mmol of 1-bromo-3-chloropropane were dissolved in 30ml of N,N-dimethylformamide, 80mmol of sodium carbonate was added, the reactant was protected by nitrogen, and stirred at 80°C under nitrogen protection Reacted for 11 hours, TLC detected that the 3-aminophenol had reacted completely, stopped the reaction, poured the reaction system into 150ml of water, extracted the generated intermediate with ethyl acetate, dried over anhydrous magnesium sulfate, removed the solvent, and obtained Intermediates were used directly in subsequent reactions.
[0042] Dissolve the intermediate obtained above into 30ml of N,N-dimethylformamide, add 80mmol of sodium carbonate, protect the reaction process with nitrogen, stir at 50 degrees Celsius under nitrogen protection and continue the reaction for 12 hours, TLC detection until the reaction is complete, the reaction After the end, the reaction system liquid was added to 100ml of water, and the target product...
Embodiment 3
[0044] 40mmol of 3-aminophenol and 240mmol of 1-bromo-3-chloropropane were dissolved in 30ml of dimethyl sulfoxide, 120mmol of sodium hydrogen phosphate was added, the reactant was protected by nitrogen, and stirred at 80°C for 10 hours under nitrogen protection , TLC detection 2-methyl-5-aminophenol reaction is complete, stop reaction, reaction system is poured into the water of 150ml, utilizes methylene dichloride to extract the intermediate that generates, then dry over anhydrous magnesium sulfate, remove solvent, The obtained intermediate was used directly in subsequent reactions.
[0045] Dissolve the intermediate obtained above into 30ml of dimethyl sulfoxide, add 120mmol sodium hydrogen phosphate, nitrogen protection during the reaction, stir at 100 degrees Celsius under nitrogen protection and continue the reaction for 12 hours, TLC detection until the reaction is complete, after the reaction, the The reaction system solution was added to 100ml of water, and the target...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com