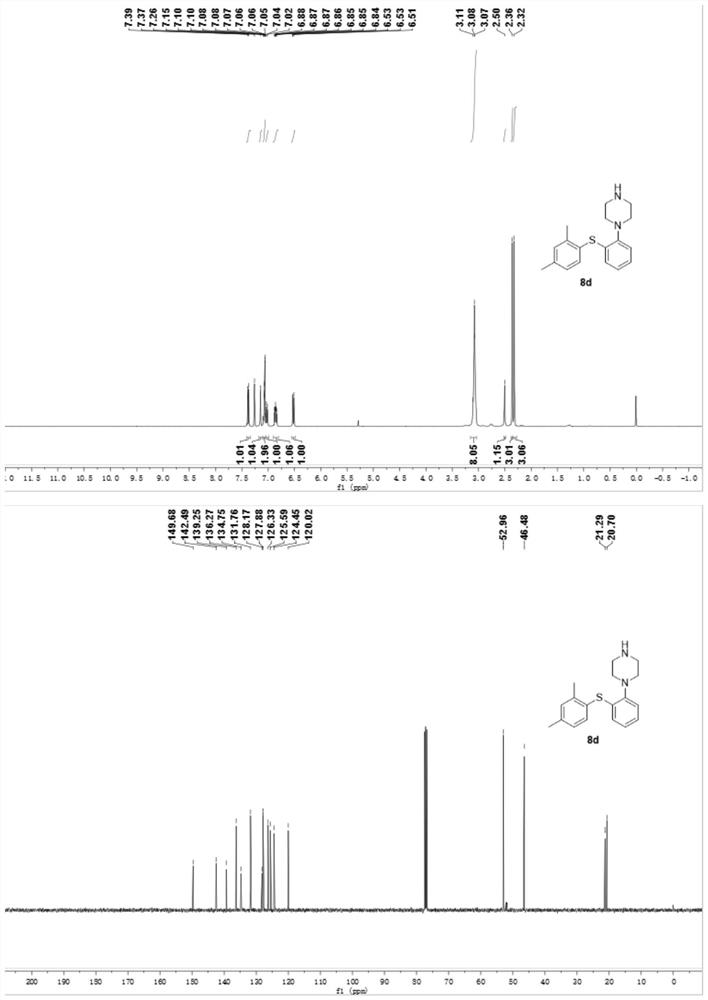
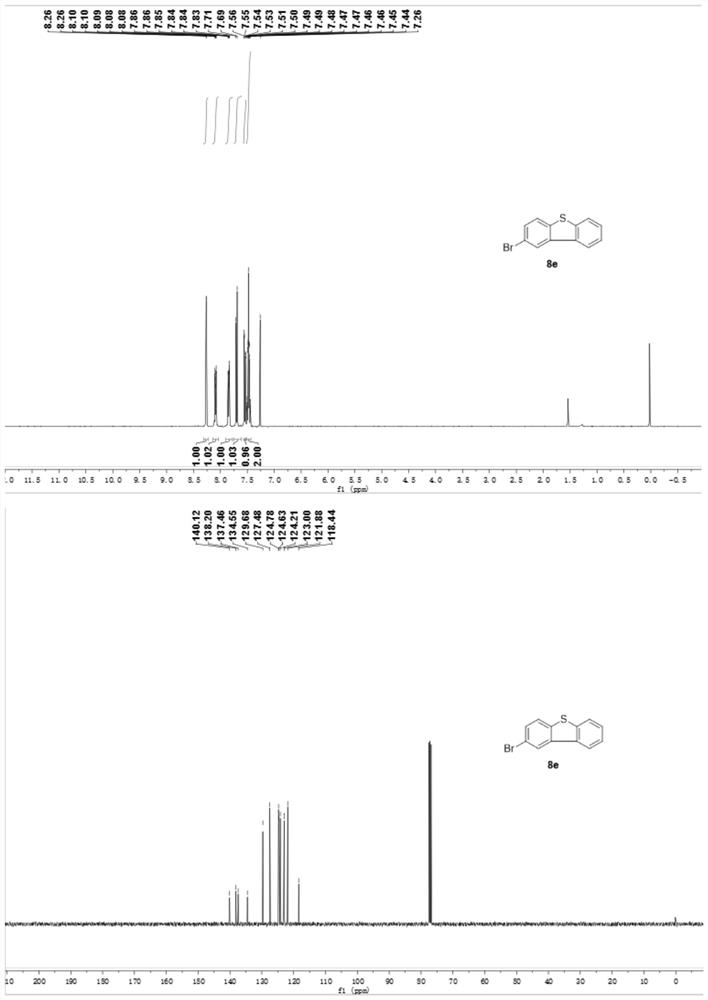
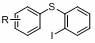
Preparation method of phenyl sulfide compound
A compound, phenylene sulfide technology, applied in the field of preparation of phenylene sulfide compounds, can solve the problems of rare transition metals, pollution, metal reagent residues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0029]
[0030] At room temperature, NaH (0.9 mmol, 3.0 equiv) was weighed in a reaction flask, suspended in anhydrous THF (0.8 mL) with conventional magnetic stirring, and thiophenol 1 (0.3 mmol, 1.0 equiv, dissolved in 0.2 mL DMA), stirred at room temperature for 2 min after the addition, then added diiodobenzene 2a (0.6 mmol, 2.0 equiv, dissolved in 0.2 mL THF), continued to stir at room temperature, and monitored the reaction by TLC. After the reaction is complete, add ice water and tetrahydrofuran to quench the reaction, extract three times with ethyl acetate, combine the organic layers, wash with saturated NaCl solution, dry over anhydrous sodium sulfate, filter, spin to dry the solvent, add silica gel powder to mix the sample, and quickly column layer Analysis and separation, the product iodine-containing phenylene sulfide 3 was obtained, and the yield was conventionally calculated.
[0031] Under the above-mentioned preparation method, thiophenol 1 with different st...
Embodiment
[0050]
[0051] Coupling reaction of phenylboronic acid with iodine-containing phenylene sulfide:
[0052]
[0053] in N 2 Under protection, iodine-containing diphenylsulfide (0.3 mmol, 1.0 equiv), phenylboronic acid (0.45 mmol, 1.5 equiv), Pd(PPh 3 ) 4 (0.03 mmol, 10 mol%), K 2 CO 3 (0.9 mmol, 3.0 equiv) was weighed into a two-necked flask, 2 mL of DMF was added, heated at 60°C for 30 min, and then the temperature was raised to 120°C for conventional stirring reaction, and the reaction was monitored by TLC. After 5 h, the raw materials were consumed. After the reaction solution was cooled to room temperature, water was added, ethyl acetate was extracted 4 times, washed 2 times with water, the organic layers were combined, washed with saturated NaCl solution, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated by rotary evaporation. The sample was mixed with silica gel powder, separated by flash column chromatography (pure PE), and the oily ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com