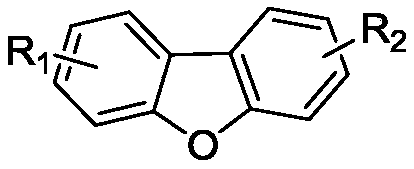
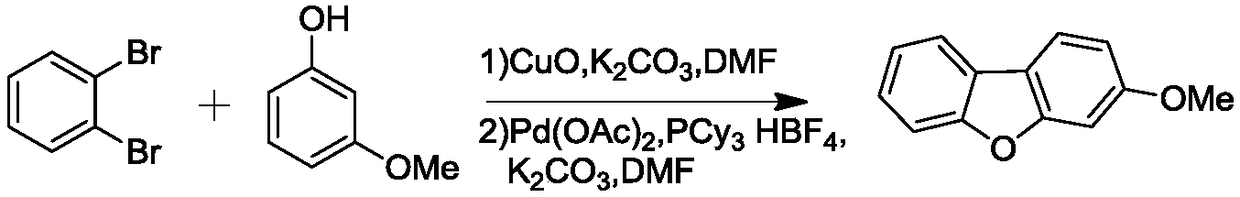Dibenzofuran derivative and preparation method thereof
A technology of furan derivatives and dibenzofuran, which is applied in the field of dibenzofuran derivatives and its preparation, can solve the problems of poor selectivity, high cost, cumbersome routes, etc., and achieve convenient post-processing, low cost and good compatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
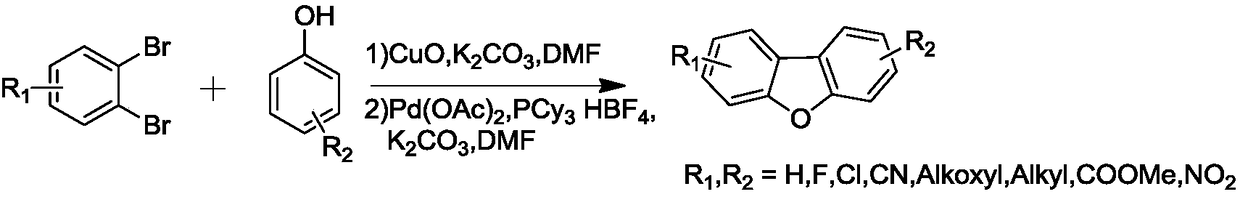
[0015] The preparation route of dibenzofuran derivatives is as follows, using ortho-dibromobenzene derivatives and phenol derivatives as starting materials, through substitution reaction and ring closure reaction process, finally generating dibenzofuran isoindigo derivatives, See Examples 1-6 for details.
[0016]
Embodiment 1
[0018] The synthetic route of 3-methoxydibenzofuran is as follows:
[0019]
[0020] Add o-dibromobenzene (0.235g, 1mmol), m-methoxyphenol (0.148g, 1.2mmol), potassium carbonate (0.069g, 0.5mmol) and copper oxide (0.079g, 1mmol) into a 25mL single-necked round bottom flask Dissolve in 5mL DMF, react under reflux for 3 hours with stirring, cool to room temperature, filter with suction, and collect the filtrate. Under nitrogen protection, the filtrate, Pd(OAc) 2 (0.011g, 5%mmol), PCy 3 ·HBF 4 (0.0368g, 10%mmol) and potassium carbonate (0.345g, 2.5mmol) were added into a 25mL three-necked flask, and the reaction system was heated to 130°C for 12 hours. After the reaction was completed, cool to room temperature, add appropriate amount of water, extract with ether, dry over anhydrous sodium sulfate, spin off the solvent, and crystallize with ether to obtain 0.18 g of white solid, with a yield of 91%. 1 H NMR (500MHz, CDCl 3 )δ7.89-7.66(m,3H),7.38-6.98(m,4H),3.83(s,3H). 13 ...
Embodiment 2
[0022] The synthetic route of 2-fluoro-7-methoxydibenzofuran is as follows:
[0023]
[0024] Add o-dibromobenzene (0.253g, 1mmol), m-methoxyphenol (0.148g, 1.2mmol), potassium carbonate (0.069g, 0.5mmol) and copper oxide (0.079g, 1mmol) into a 25mL single-necked round bottom flask Dissolve in 5mL DMF, react under reflux for 3 hours with stirring, cool to room temperature, filter with suction, and collect the filtrate. Under nitrogen protection, the filtrate, Pd(OAc) 2 (0.011g, 5%mmol), PCy 3 ·HBF 4 (0.0368g, 10%mmol) and potassium carbonate (0.345g, 2.5mmol) were added into a 25mL three-necked flask, and the reaction system was heated to 130°C for 12 hours. After the reaction was completed, cool to room temperature, add appropriate amount of water, extract with ether, dry over anhydrous sodium sulfate, spin off the solvent, and crystallize with ether to obtain 0.18 g of white solid with a yield of 83%. 1 H NMR (500MHz, CDCl 3 )δ7.78-7.64(m,2H),7.52-7.35(m,2H),7.22-6.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com