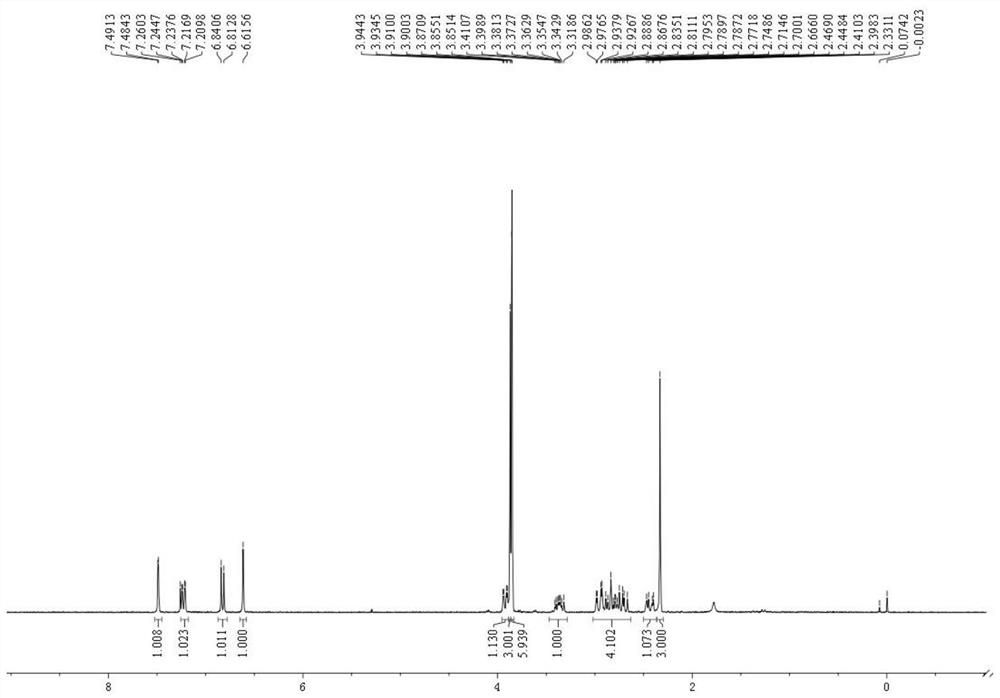
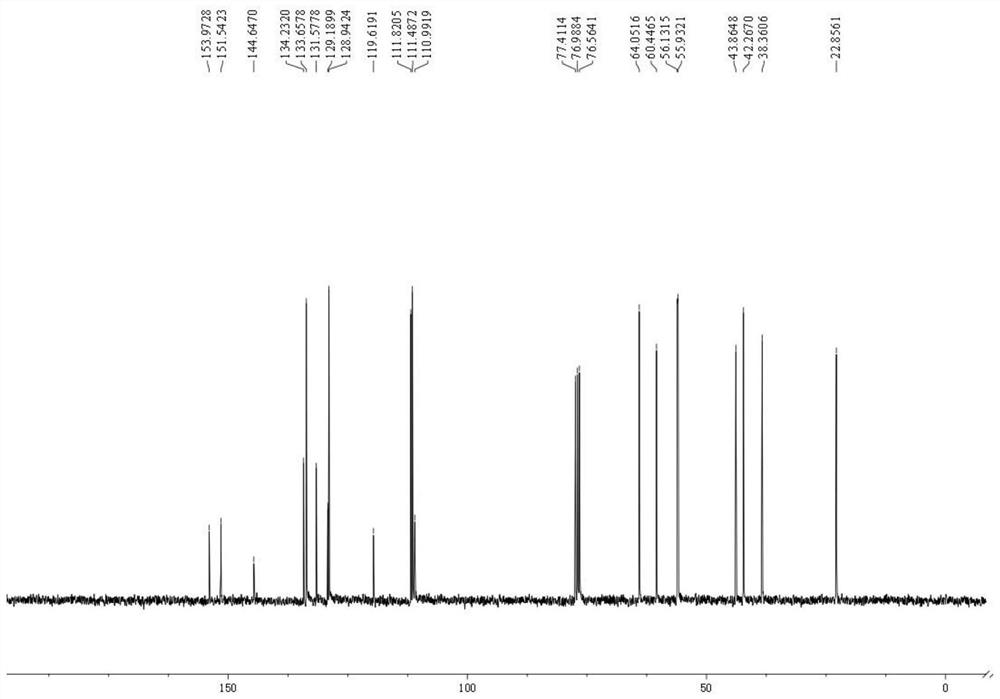
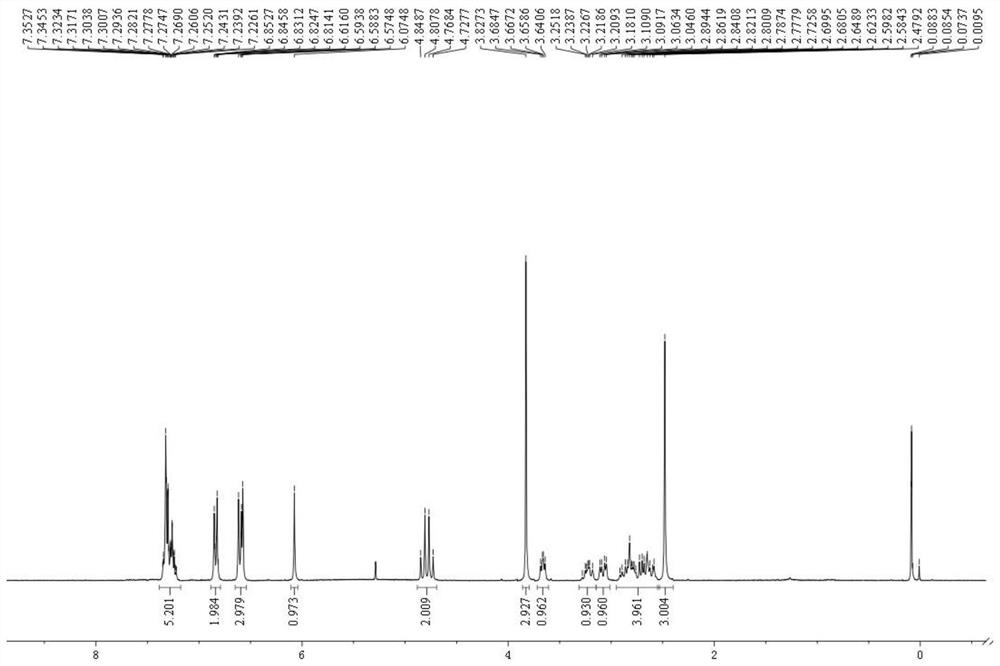
Novel total synthesis method of racemic tetrandrine
A total synthesis technology of tetrandrine, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of risk, high synthesis cost, short route, etc., and achieve the effect of improved synthesis efficiency, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] (1) Preparation of compound 2
[0073] Add 100g of 5-bromovanillin, 8g of potassium iodide, 30g of sodium hydroxide and 800g of dichloromethane into a 2L three-necked flask, and add 96g of benzyl bromide dropwise at room temperature. After dropping, heat to reflux for 3 hours, and the reaction of the raw materials is complete. Cool down to room temperature, filter, and wash the filtrate twice with 800 g of 5% sodium hydroxide solution, once with 400 g of water, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain a yellow oil, which was crystallized by 400 g of petroleum ether to obtain 124 g of a white solid, which was compound 2, with a yield of 89.2%;
[0074] (2) Preparation of compound 3
[0075] Add 100g of compound 2, 36g of triethylamine, 57g of nitromethane and 500g of ethanol into a 2L three-neck flask, heat to reflux for 12 hours, and the reaction of the raw materials is complete....
Embodiment 2
[0106] (1) Preparation of compound 2
[0107] 120g of 5-bromovanillin, 12g of potassium iodide, 72g of potassium carbonate and 800g of ethanol were added to a 2L three-necked flask, and 115g of p-methoxybenzyl bromide was added dropwise at room temperature. After dropping, heat to reflux for 6 hours, and the reaction of raw materials is complete. Cool down to room temperature, filter, and concentrate the filtrate to dryness under reduced pressure, add 600g of dichloromethane to dissolve, wash the organic phase twice with 800g of 5% sodium hydroxide solution, once with 400g of water, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain a yellow oil, which was crystallized from 480 g of petroleum ether to obtain 150 g of a white solid, namely compound 2, with a yield of 82.1%;
[0108] (2) Preparation of compound 3
[0109] 110g of compound 2, 67g of ammonium acetate and 330g of nitromethane were add...
Embodiment 3
[0139] (1) Preparation of compound 2
[0140] Add 115g of 5-bromovanillin, 75g of triethylamine and 675g of acetonitrile into a 2L three-necked flask, cool down to 0-5°C, and add 95g of p-toluenesulfonyl chloride in batches. After the addition was completed, the insulation reaction was carried out for 3 hours, and the reaction of the raw materials was complete. The reaction solution was concentrated to dryness under reduced pressure, 300 g of water and 300 g of dichloromethane were added, the pH was adjusted to about 3 with 12% hydrochloric acid, the liquid was separated after standing, the organic phase was washed twice with 600 g of water, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure, and after purification by 675 g of ethanol, 168 g of an off-white solid was obtained, which was compound 2, with a yield of 87.4%;
[0141] (2) Preparation of compound 3
[0142] Add 96g of compound 2, 12g of sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com