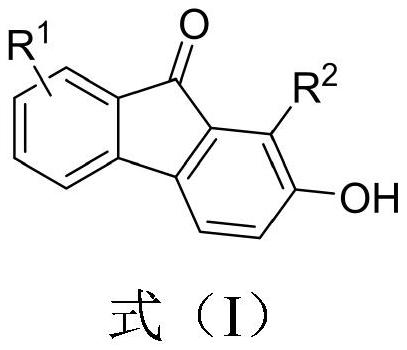
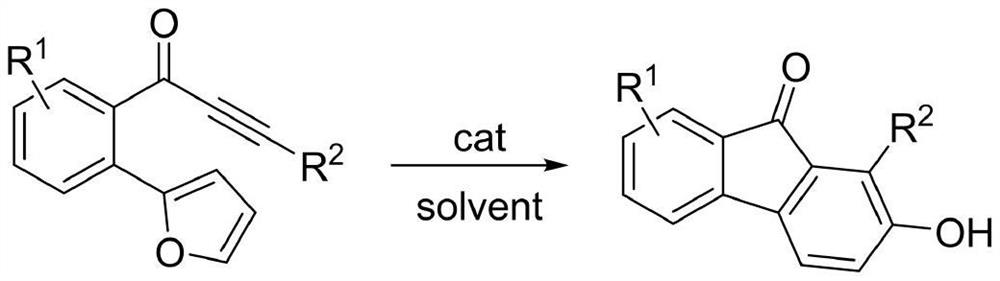
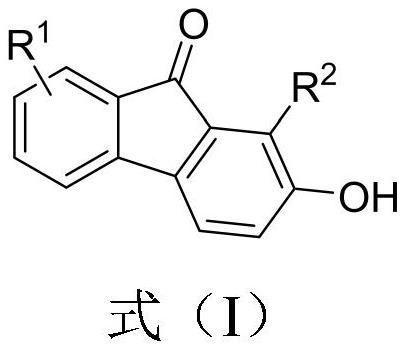
2-hydroxy-9-fluorenone derivative as well as synthesis method and application thereof
A synthesis method and derivative technology, applied in the field of 2-hydroxy-9-fluorenone compounds and their synthesis, can solve the problems of poor functional group tolerance, complex ligands, low product yield, etc., and achieve substrate universality Good, mild reaction conditions, simple post-treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Synthesis of 1-phenyl-2-hydroxyl-9-fluorenone (IA)
[0029]
[0030]Under air (without inert gas protection), 0.3 mmol of o-furyl phenyl ynone, 3 mL of toluene, and 0.03 mmol of p-toluenesulfonic acid monohydrate were added to a 10 ml sealed tube. React at 100°C for 17 hours, then cool to room temperature, concentrate, and perform column chromatography to obtain the target product formula (IA), a red solid, with an isolation yield of 95%. MP 194-196°C.
[0031] Product NMR data: 1H NMR (CDCl 3 ,400MHz): δ5.32(s,1H),7.07(d,J=8.0Hz,1H),7.17(d,J=3.2Hz,1H),7.37-7.43(m,5H),7.47-7.54( m,5H); 13C NMR (CDCl 3 ,100MHz): δ119.526, 120.34, 120.84, 124.06, 127.61, 127.92, 128.98, 129.15, 129.62, 131.21, 131.60, 134.14, 134.63, 137.45, 144.05, 154.15, 192.9 z) calcd. for C 19 h 12 o 2 [M+H]+calc.: 273.0916; found: 273.0920.
Embodiment 2
[0032] Example 2: Synthesis of 1-phenyl-2-hydroxyl-6-chloro-9-fluorenone (IB)
[0033]
[0034] Under air (without inert gas protection), 0.3 mmol of chloro-substituted o-furaryl acetylenone, 3 mL of toluene, and 0.03 mmol of p-toluenesulfonic acid monohydrate were added to a sealed 10 ml tube. React at 100°C for 18 hours, then cool to room temperature, concentrate, and perform column chromatography to obtain the target product formula (IB), a dark red solid, with an isolation yield of 90%. MP 206-207°C.
[0035] Product NMR data: 1H NMR [(CD 3 ) 2 SO,400MHz]:δ7.09(d,J=8.0Hz,1H),7.27-7.31(m,3H),7.36-7.39(m,4H),7.66(d,J=8.0Hz,1H),7.80 (d, J=1.6Hz, 1H), 10.03(s, 1H); 13CNMR[(CD 3 ) 2 SO,100MHz]:δ120.11,120.44,121.77,124.92,127.28,127.43,128.92,130.03,131.56,132.02,133.09,133.97,139.80,145.87,156.86,190.99.
[0036] High resolution mass spectrometry data: HRMS(ESI,m / z)calcd.for C 19 h 11 ClO 2 [M+H]+calc.: 307.0520; found: 307.0512.
Embodiment 3
[0037] Example 3: Synthesis of 1-phenyl-2-hydroxyl-6-fluoro-9-fluorenone (IC)
[0038]
[0039] Under air (without inert gas protection), 0.3 mmol of fluorine-substituted o-furaryl acetylenone, 3 mL of toluene, and 0.03 mmol of p-toluenesulfonic acid monohydrate were added to a 10 ml sealed tube. React at 100°C for 18 hours, then cool to room temperature, concentrate, and perform column chromatography to obtain the target product formula (IC), a red solid, with an isolation yield of 100%. MP 210-213°C.
[0040] Product NMR data: 1H NMR [(CD 3 ) 2 SO,400MHz]:δ6.97-7.02(m,1H),7.09(d,J=8.0Hz,1H),7.30-7.34(m,2H),7.35-7.41(m,3H),7.43(dd, J 1 =8.2Hz,J 2 =5.6Hz,1H),7.56(dd,J 1 =9.0Hz,J 2 =2.0Hz, 1H), 7.62(d, J=8.0Hz, 1H), 10.02(s, 1H); 13C[(CD 3 ) 2 SO,100MHz]:δ107.73(d,J=24.6Hz),114.01(d,J=23.4Hz),120.27,121.66,125.84(d,J=10.4Hz),127.22,127.27,128.78,129.82,130.06 ,131.85,133.12,133.76,147.12(J=10.6Hz),156.84,166.85(d,J=250Hz),190.65.
[0041] High resolution mass spec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Separation yield | aaaaa | aaaaa |
| Separation yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com