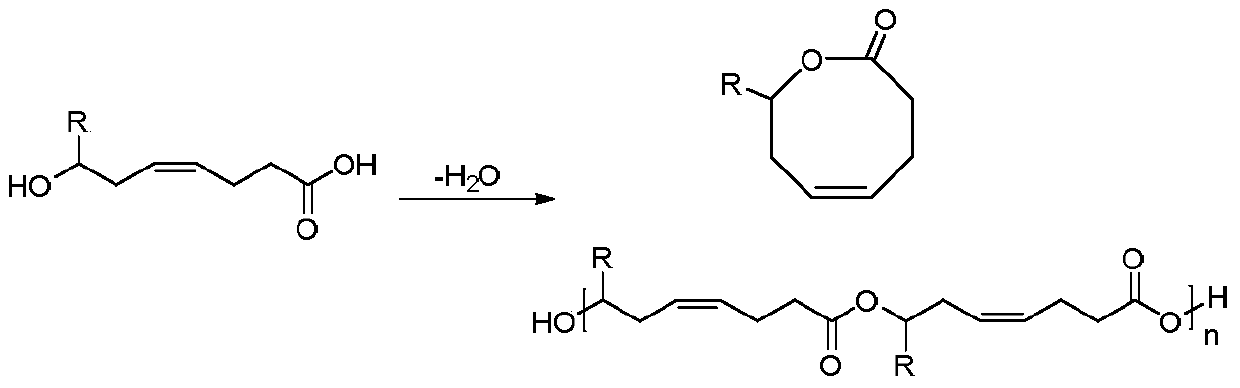
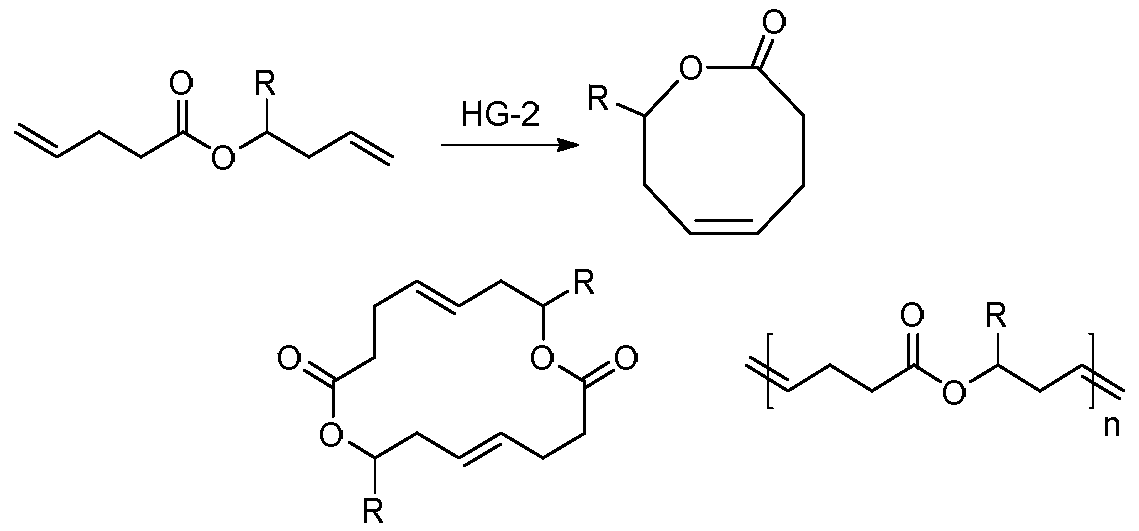
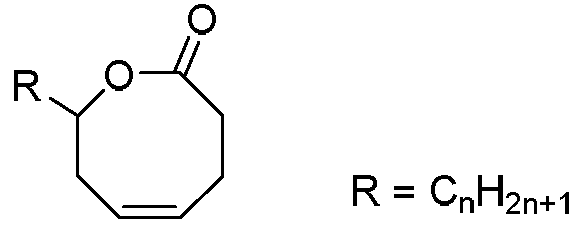Method for synthesizing 8th-site substituted-3,4,7,8-tetrahydro-2H-oxocin-2-one octatomic ring
A synthesis method and an eight-membered ring technology, applied in the direction of organic chemistry, etc., can solve the problems of easy generation of a large number of 16-membered ring dimers, difficult removal, inability to obtain, etc., achieving easy operation, wide applicability, and simple process conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] 1. Synthetic allyl alcohol compounds, the operating process is as follows:
[0019] Under argon gas, add various aldehydes or ketones (1.0eq.) to a dry round bottom flask, dissolve them in anhydrous ether (1mL / mmol) solvent, and control the temperature according to the steric hindrance of aldehydes and ketones. 0-25°C, extract the freshly prepared Grignard reagent (3.0eq.) and slowly drop it into the flask. After addition, stir at room temperature for 15h. After the reaction was completed, the saturated ammonium chloride solution was quenched until the solution was transparent, extracted with ethyl acetate and saturated brine, the organic layer was dried with anhydrous sodium sulfate, filtered and rotary evaporated, and separated by column chromatography to obtain the corresponding allyl alcohol compounds .
[0020] This step reaction formula is as follows:
[0021]
[0022] Compound NMR spectrum:
[0023] 1-phenyl-3-en-1-ol: 62%, 1 H NMR (400MHz, CDCl 3 ) δ 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com