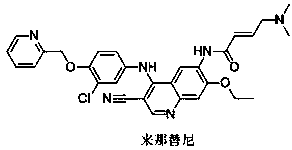
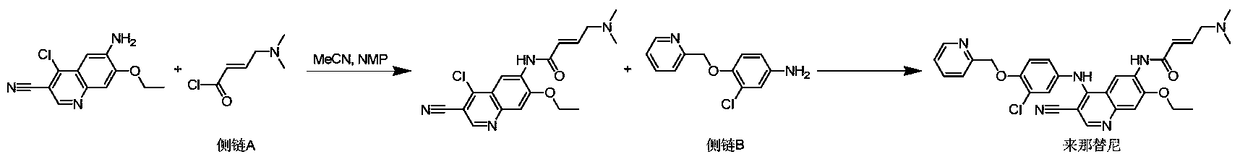
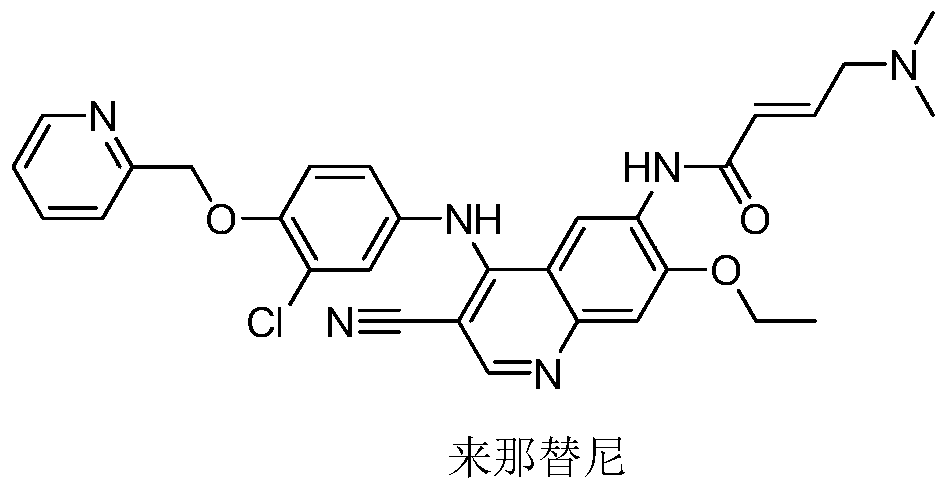
A novel preparation method of epidermal growth factor receptor (EGFR) inhibitor neratinib
A technology of epidermal growth factor and neratinib, applied in the field of organic synthesis, can solve the problems of poor solubility of intermediates, large amount of solvent and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 0.4L of dimethyl sulfoxide to a 2L three-necked flask, add 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro- 3-quinolinecarbonitrile (I) (43.06g, 0.12mol, 1eq) and 2-chloro-4-aminophenol (II) (17.23g, 0.12moL, 1eq), nitrogen replacement three times, the reaction system was heated to 90 ℃, add dichloromethylpyridine hydrochloride (Ⅲ) (29.53g, 0.18moL, 1.50eq) dissolved in tetrahydrofuran 0.15L dropwise at a constant speed, add dropwise for 1 hour, keep warm for 2 hours after the dropwise addition, monitor by HPLC 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro-3-quinolinecarbonitrile (Ⅰ)≤0.2%, the reaction system is cooled To room temperature, potassium carbonate (66.34g, 0.48moL, 4eq) and sodium iodide (1.80g, 0.01moL, 0.1eq) were added, the temperature of the reaction system was raised to 90°C, and the reaction was kept for 16 hours, and the intermediate state (E) was monitored by HPLC -N-{4-[3-chloro-4-hydroxyanilino]3-cyano-7-ethoxy-6-quinoline}...
Embodiment 2
[0025] Add 300 mL of tetrahydrofuran into a 2L three-necked flask, add 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro-3-quinoline Nitrile (I) (28.70g, 0.08mol, 1eq) and 2-chloro-4-aminophenol (II) (11.49g, 0.08mol, 1eq), replaced by nitrogen three times, the reaction system was heated to 65 ° C, and added dropwise Dichloromethylpyridine hydrochloride (Ⅲ) (13.78g, 0.08moL, 1.05eq) dissolved in 100mL of tetrahydrofuran was added dropwise for 1 hour. After the addition was completed, the reaction was incubated for 2 hours. HPLC monitored 6-[(E) -4-(dimethylamino)-2-butenylamino]-7-ethoxy-4-chloro-3-quinolinecarbonitrile (Ⅰ)≤0.5%, the reaction system was cooled to room temperature, and potassium carbonate was added (44.23g, 0.32moL, 4eq) and potassium iodide (1.99g, 0.01moL, 0.1eq), the temperature of the reaction system was raised to 60°C, and the reaction was kept for 16 hours. The intermediate state (E)-N-{4-[3 -Chloro-4-hydroxyanilino]3-cyano-7-ethoxy-6-quinoline}-4...
Embodiment 3
[0027] Add 300 mL of tetrahydrofuran into a 2L three-necked flask, add 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro-3-quinoline Nitrile (I) (35.88g, 0.1mol, 1eq) and 2-chloro-4-aminophenol (II) (14.36g, 0.1moL, 1eq), replaced by nitrogen three times, the temperature of the reaction system was raised to 60°C, and the Dichloromethylpyridine hydrochloride (Ⅲ) (17.22g, 0.11moL, 1.05eq) dissolved in 100mL of tetrahydrofuran was added dropwise for 1 hour. After the dropwise addition was completed, the reaction was incubated for 2 hours. HPLC monitored 6-[(E) -4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro-3-quinolinecarbonitrile (Ⅰ)≤0.3%, the reaction system was cooled to room temperature, and sodium methoxide was added (10.80g, 0.20moL, 2eq) and sodium iodide (1.5g, 0.01moL, 0.1eq), the temperature of the reaction system was raised to 60°C, and the reaction was kept for 16 hours. The intermediate state (E)-N-{4- [3-Chloro-4-hydroxyanilino]3-cyano-7-ethoxy-6-quinolin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com