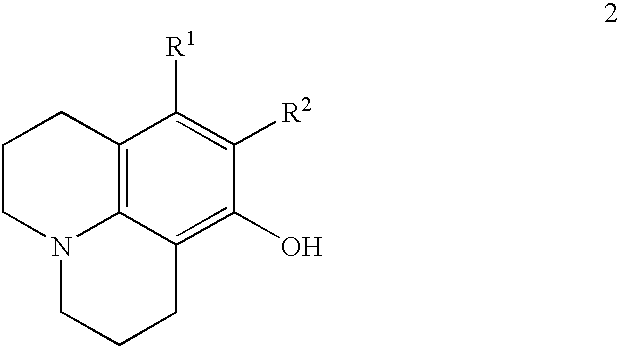
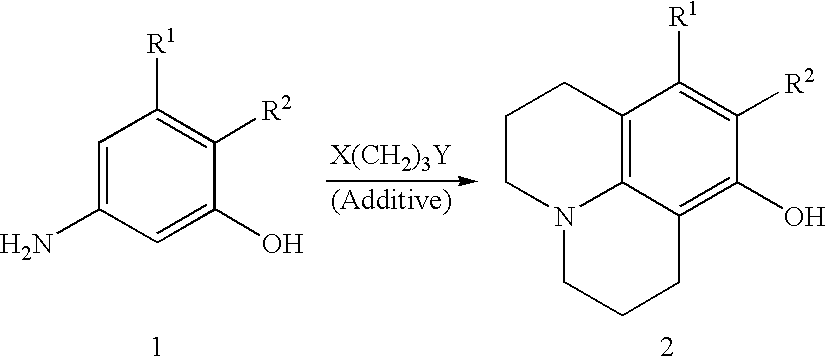
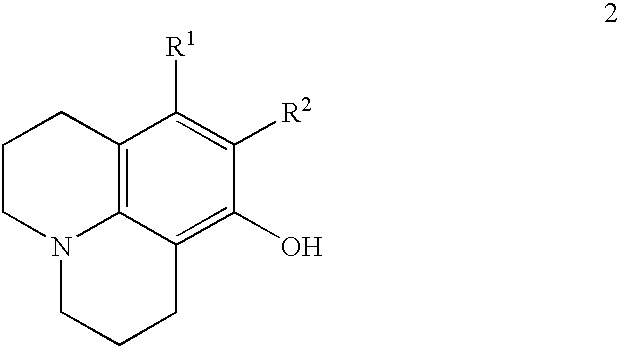
Method for making 8-hydroxyjulolidine compound
a technology of julolidine and compound, applied in the field of making 8-hydroxyjulolidine compound, can solve the problems of reducing energy dissipation caused, bringing out relatively higher production costs, and hazardous materials to the environment, and achieves the effect of easy preparation and higher yield of the invented method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
8-Hydroxyjulolidine
[0022] m-Aminophenol (110 mg, 1 mmol), 1-bromo-3-chloropropane (2.35 g, 1.6 ml, 15 mmol) and anhydrous Na.sub.2CO.sub.3 (212 g, 2 mmol) were placed in a 100 ml two-necked round-bottomed flask equipped with a thermometer and an addition funnel containing molecular sieves (4.ANG., 0.3 g). The top of the funnel was fitted with a condenser. Under an atmosphere of nitrogen, the mixture was heated to 70.degree. C. for 3 hours and then refluxed at 110.degree. C. for 15 hours. The resulting red mixture was cooled to room temperature and concentrated HCI solution (15 ml) was slowly added with care. After addition of CH.sub.2Cl.sub.2 (5 ml), the aqueous layer was separated. The aqueous phase was neutralized by the addition of 40% NaOH aqueous solution and extracted with CH.sub.2Cl.sub.2 (50 ml.times.4). The CH.sub.2Cl.sub.2 extracts were combined, washed with brine, dried over anhydrous MgSO.sub.4 and concentrated in reduced pressure. The crude product was subjected to sili...
embodiment 2
8-Hydroxyjuloldine
[0024] According to a procedure similar to that of Embodiment 1, a mixture of m-aminophenol(1.1 g, 10 mmol), 1-bromo-3-chloropropane (23.5 g, 16 ml, 150 mmol) and anhydrous Na.sub.2CO.sub.3 (4.27 g, 40 mmol) was heated and stirred at 70.degree. C. for 3 hours and refluxed at 110.degree. C. for 15 hours. After silica gel column chromatography 8-hydroxyjulolidine (800 mg) was obtained in 42% yield.
embodiment 3
8-Hydroxyjulolidine
[0025] According to a procedure similar to that of Embodiment 1, a mixture of m-aminophenol (11.0 g, 101 mmol), 1 -bromo-3-chloropropane (127.4 g, 80 ml, 809 mmol) and anhydrous Na.sub.2CO.sub.3 (42.4 g, 400 mmol) was heated and stirred at 70.degree. C. for 3 hours and refluxed at 110.degree. C. for 15 hours. After silica gel column chromatography, 8-hydroxyjulolidine (7.3 g) was obtained in 38% yield.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| chemical structure | aaaaa | aaaaa |
| phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com