Method for synthesizing doxylamine succinate
A technology for the synthesis of doxylamine succinate and its synthesis method, which is applied in the field of synthesis of ethanol antihistamines and can solve the problems of no literature reports on doxylamine succinate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
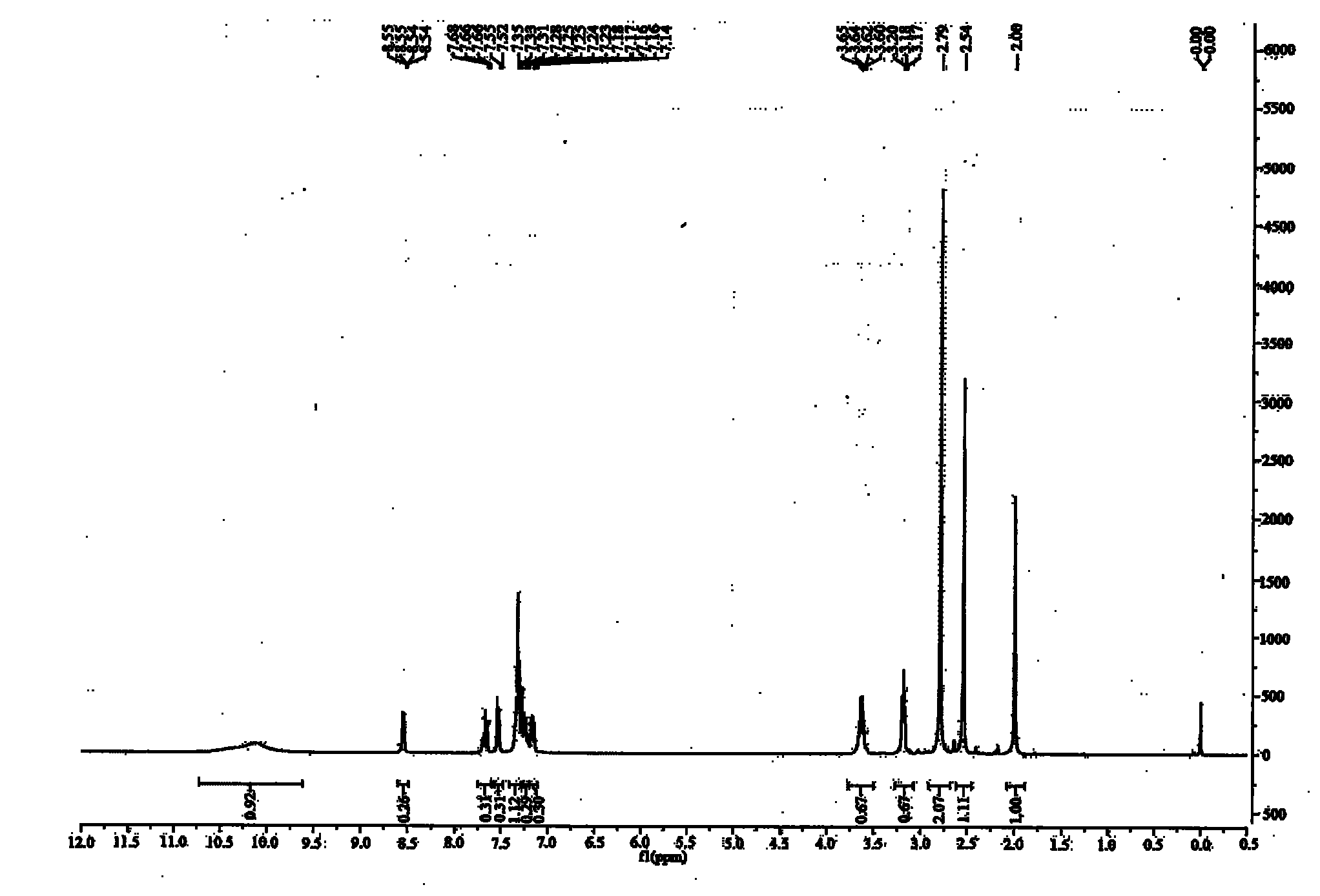
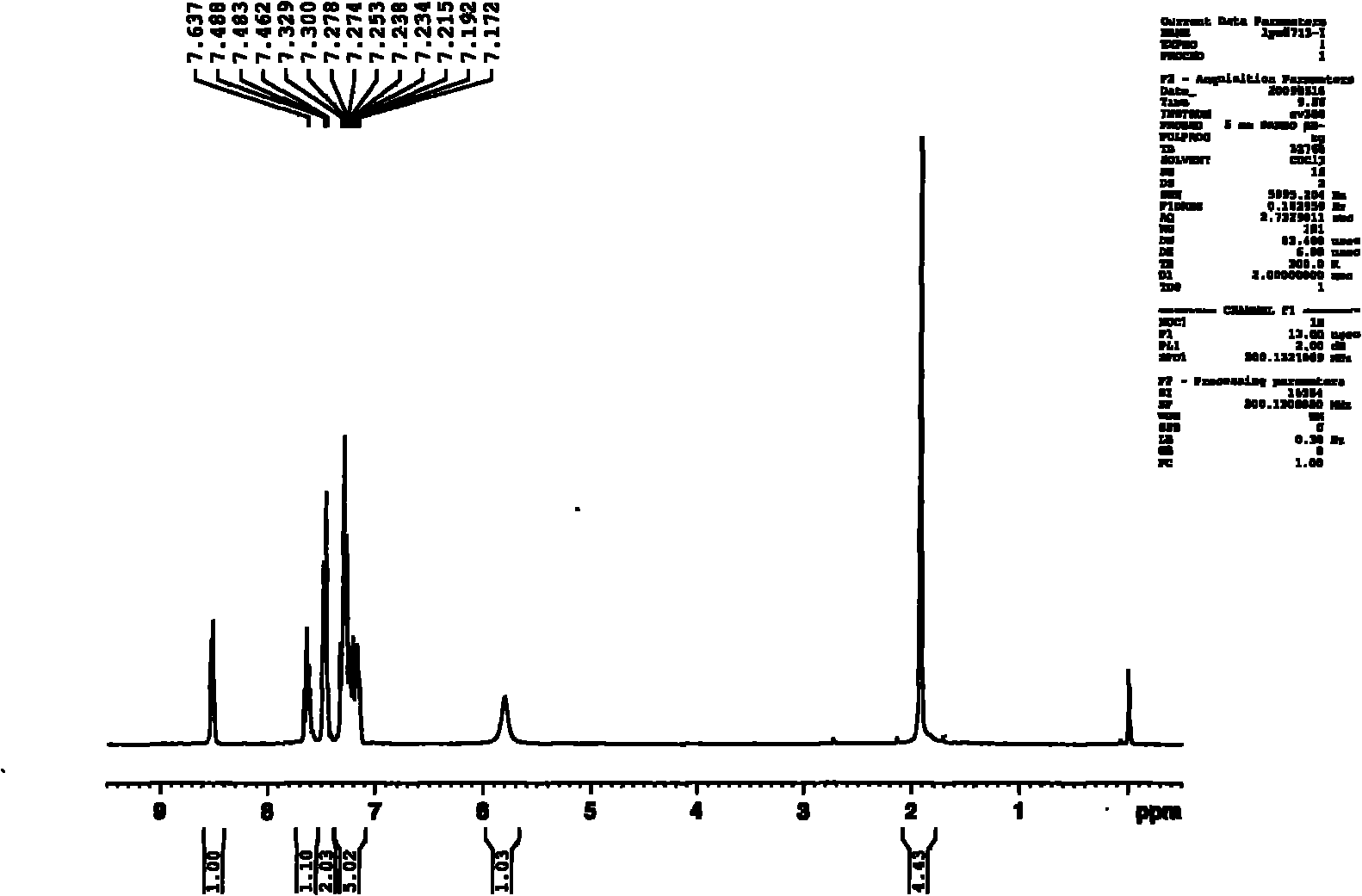
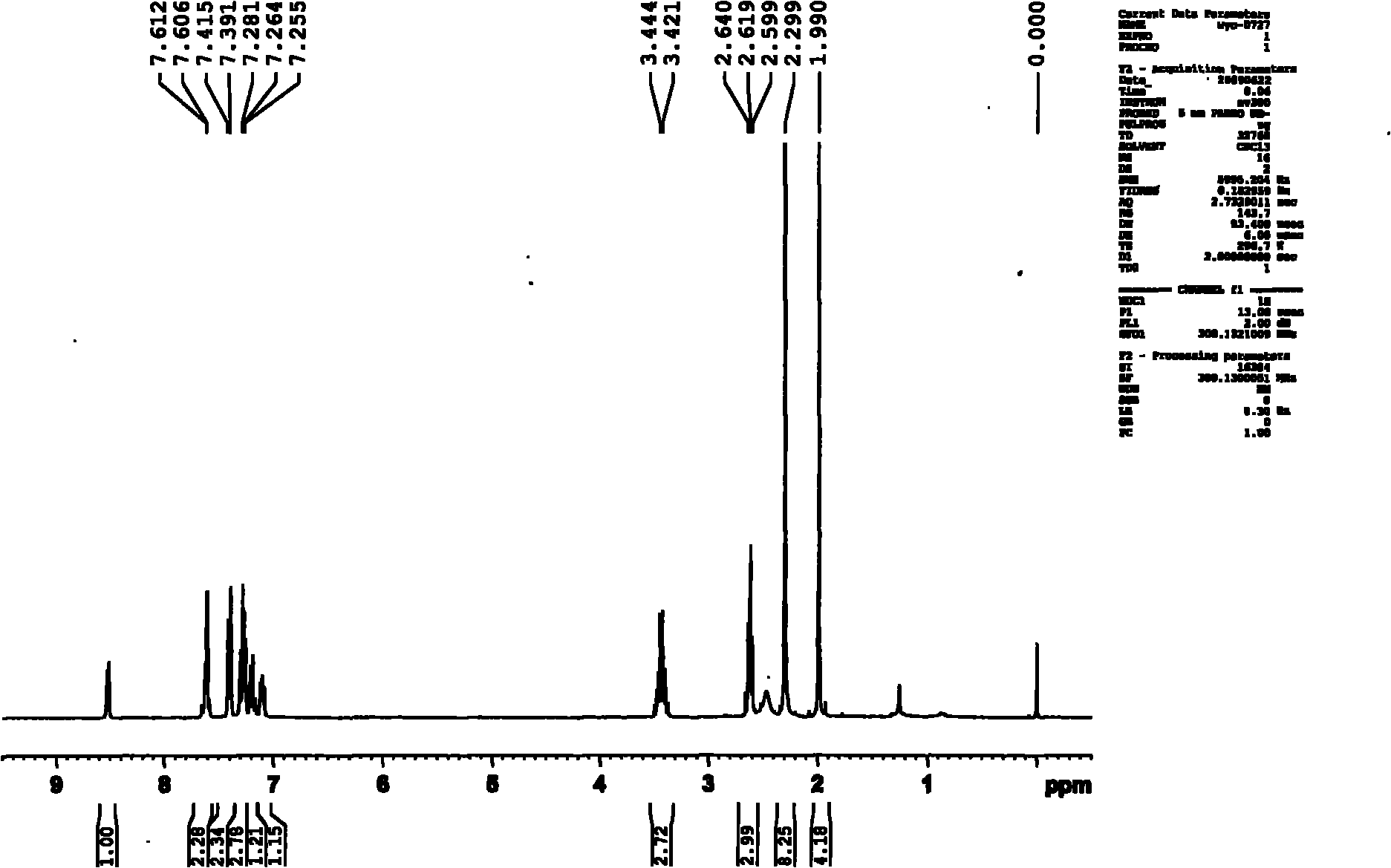
[0033] 1. Synthesis of 2-pyridylphenylmethylcarbinol
[0034] Take 3.12g (0.13mol) of magnesium chips into a 250ml three-neck flask, add 50ml of anhydrous ether, stir, take 17.27g (0.11mol) of bromobenzene and mix 50ml of anhydrous ether, and slowly drop them. About 10 minutes to drip. After the reaction was initiated, a large amount of diethyl ether was refluxed, and the reaction solution became cloudy and the color gradually turned to metallic gray. Keep ether at reflux for two hours. Take 12.1g (0.1mol) of 2-acetylpyridine, mix with 30ml of anhydrous diethyl ether, and slowly add it dropwise to the above-mentioned Grignard reagent, and the dropping rate is controlled at 2 seconds per drop. When the mixture was added to Grignard reagent, it immediately turned into a white milky substance, and a large amount of heat was released, and anhydrous ether was refluxed. After the dropwise addition, the solution became clear, and a gray sticky substance appeared at the bottom of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com