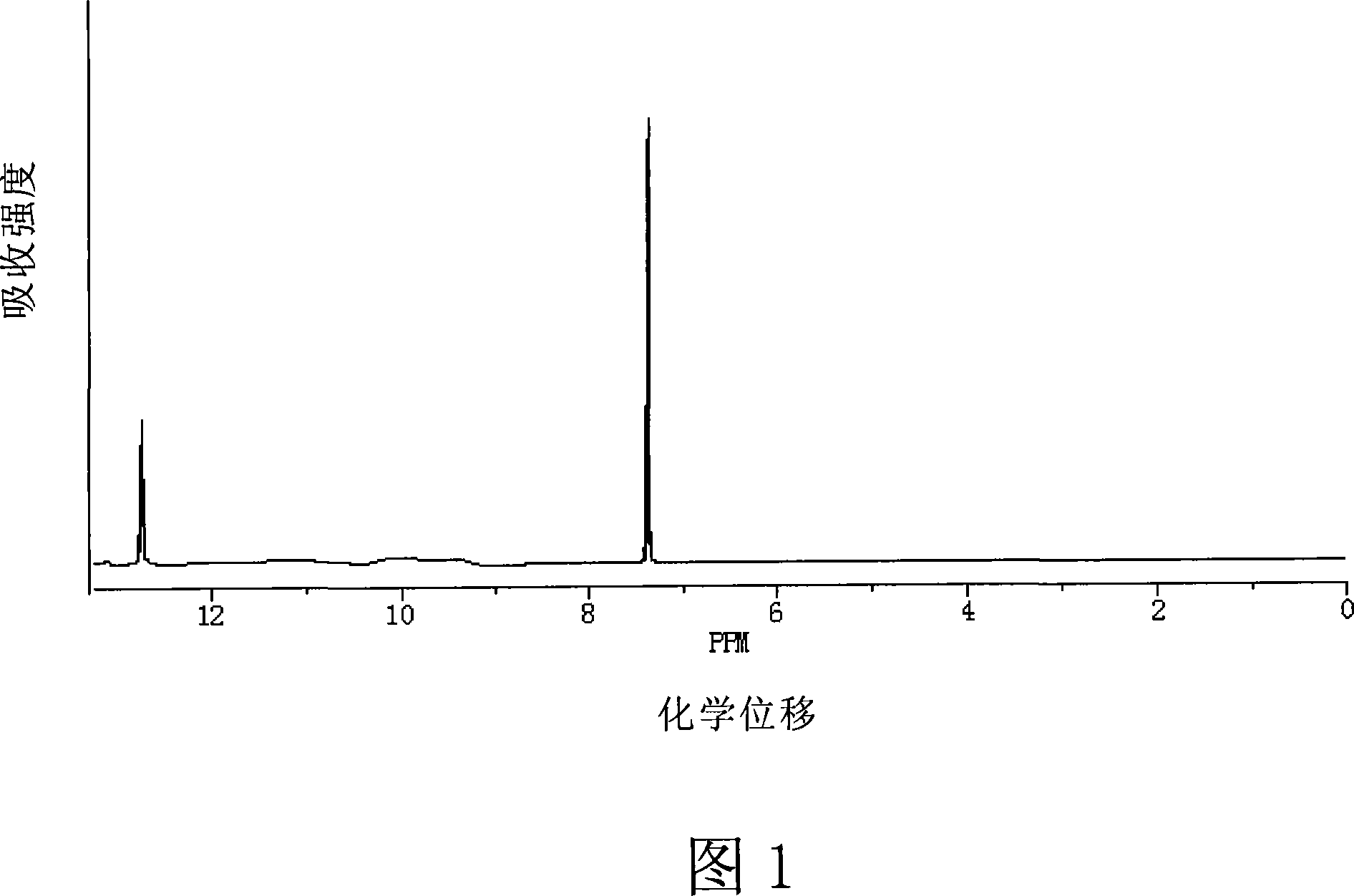
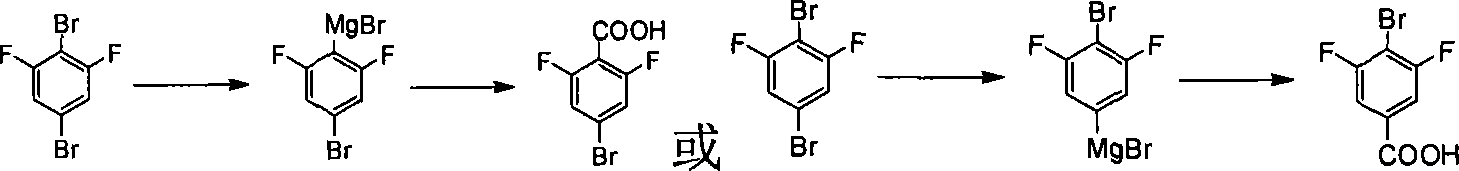
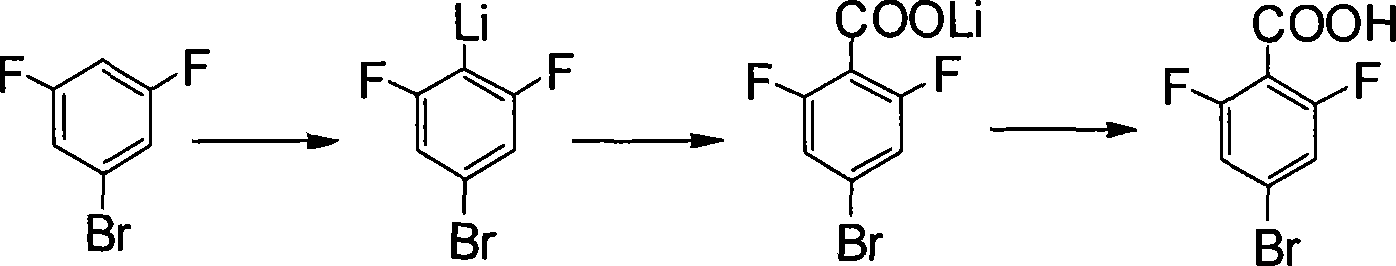
Method for preparing 4 - bromine 2,6 - difluoro benzoic acid
A technology of difluorobenzoic acid and difluorobromobenzene, which is applied in the field of organic chemical synthesis, and can solve the problems of large environmental pollution, difficulty in industrialization, and difficulty in synthesizing final products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The preparation method of the present invention is described in detail below:
[0059] The steps of the preparation method of 4-bromo-2,6-difluorobenzoic acid
[0060] The preparation method of 4-bromo-2,6-difluorobenzoic acid of the present invention comprises the following steps:
[0061] (a) 3,5-difluorobromobenzene reacts with organolithium reagent in the presence of organic solvent to obtain the 4-position substituted lithium salt of 3,5-difluorobromobenzene;
[0062] (b) The 4-position substituted lithium salt of 3,5-difluorobromobenzene obtained in step (a) is subjected to hydrolysis to obtain 4-bromo-2,6-difluorobenzoic acid.
[0063] In a preferred embodiment of the present invention, the preparation method of 4-bromo-2,6-difluorobenzoic acid of the present invention comprises the following steps:
[0064] (a) 3,5-difluorobromobenzene reacts with an organolithium reagent in the presence of an organic solvent to obtain a 4-position substituted lithium salt of ...
Embodiment 1
[0098] Add 300ml THF (molecular weight 72.11, density 0.8892, 3.7mol), 10% butyllithium solution 0.39mol (250g) and 2,2,6,6-tetramethylpiperidine (0.39mol 56g) into a 1L bottle, Add 0.3 mol (58 g) of 3,5-difluorobromobenzene (99.8%) dropwise at -10°C (the molar ratio of solvent to 3,5-difluorobromobenzene is 12.3:1), stir for about 30 min, and cool to - 90℃ through CO 2 gas, to saturation. CO 2 After saturation, the temperature rises naturally. When rising to the internal temperature of -10°C, add 400ml of dilute hydrochloric acid, to PH<1, separate the upper organic layer, the aqueous layer is extracted with ether, the organic layer is washed with water, dried, and evaporated to remove the solvent to obtain 71 grams of the product, the product is cream color. After recrystallization, 59 grams of white product was obtained, the HPLC content was 99.4%, and the molar yield of raw material to product=88.9%.
Embodiment 2
[0100] Add 400ml of ether (molecular weight 74.12, density 0.7135, 3.85mol), 10% butyllithium solution 0.15mol (96g) and 0.15mol (18g) tetramethylethylenediamine to a 1L bottle, add 3 dropwise at 50°C, 0.3mol (58g) of 5-difluorobromobenzene (molar ratio of solvent to 3,5-difluorobromobenzene is 12.8:1), stirred for about 30min, cooled to 10°C and passed through CO 2 gas, to saturation. CO 2 After saturation, the temperature rises naturally. When the internal temperature was raised to 30°C, 400ml of dilute hydrochloric acid was added, to pH<1, the upper organic layer was separated, the aqueous layer was extracted with ether, the organic layer was washed with water, dried, and the solvent was evaporated to obtain 58 grams of the product, which was beige color. After recrystallization, 49 grams of white product were obtained, and the HPLC content was 99.0%. Molar yield of feedstock to product = 67.8%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com