One-pot synthesis method for substituted indane compounds
The technology of a compound, dihydroindane, is applied in the field of synthesis of pharmaceutical intermediates, which can solve the problems of low yield and cumbersome reaction process, and achieve the effects of shortened routes, mild reaction conditions, and shortened labor time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
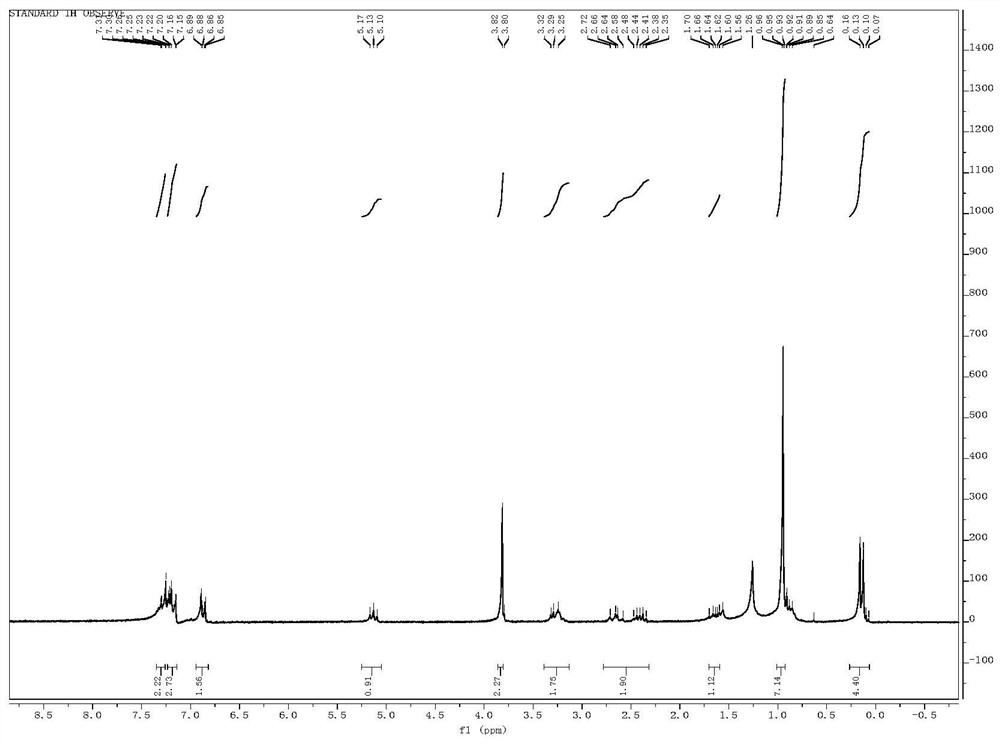
[0036] Example 1 Synthesis of 1-(4-methoxybenzyl)-2,3-dihydro-1H-indene (1)
[0037]
[0038] Slowly add the compound 1-(3-ene-butyl )-2-iodobenzene (633mg, 3mmol) and PEPPSI (40.8mg, 0.06mmol) in 3mL THF mixed solution, the reaction solution was stirred at room temperature for 5 minutes. The reaction solution was saturated with NH 4 Cl solution was quenched, extracted with ethyl acetate, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, the organic phase was concentrated, and the crude product was separated by column chromatography (eluent: PE:EA=100:1) to obtain compound 1 (489mg, 85%) as a colorless oil. 1 H NMR (CDCl 3 ,400MHz,ppm):δ=7.23-6.83(m,8H),3.81(s,3H), 3.46-3.36(m,1H),3.07(dd,J=13.7Hz; 5.8Hz,1H),2.93- 2.74 (m,2H),2.65(dd,J=13.7Hz;9.3Hz,1H),2.20-2.09(m,1H), 1.82-1.69(m,1H).
Embodiment 2
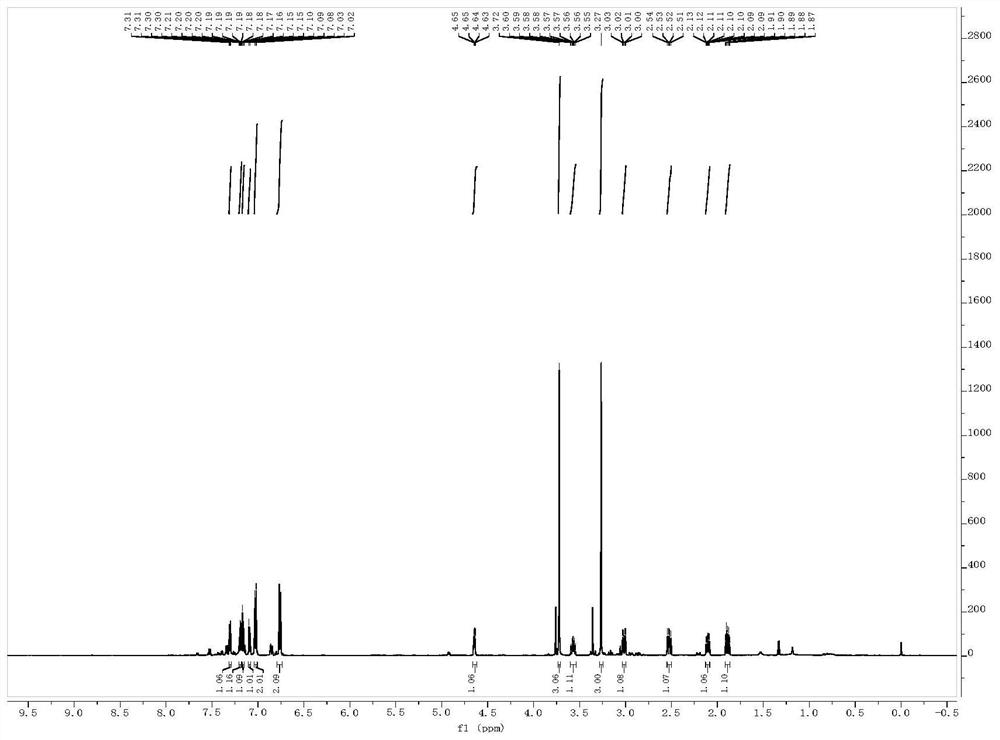
[0039] Example 2 Synthesis of tert-butyl((3-(4-methoxybenzyl)-2,3-dihydro-1H-inden-1-yl)oxy)dimethylsilane(2)
[0040]
[0041] The compound tert-butyl ((1-(2- A mixed solution of iodophenyl)but-3-en-1-yl)oxy)dimethylsilane (250mg, 0.64mmol) and PEPPSI (17.4mg, 0.025mmol) in 4mL THF was stirred at room temperature for 5 minutes. The reaction solution was saturated with NH 4 The Cl solution was quenched, extracted with ethyl acetate, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, the organic phase was concentrated, and the crude product was separated by column chromatography (eluent: PE:EA=100:1) to obtain compound 2 (114mg, 50%) as a colorless oil. 1H NMR (CDCl 3 ,400MHz,ppm): δ=7.28-6.76(m,8H),5.01(t,J=7.4Hz,1H),3.79(s,3H), 3.20-2.92(m,3H),2.10-1.54(m ,2H),0.98(s,9H),0.21(s,6H).
Embodiment 3
[0042] Example 3 Synthesis of 1-methoxy-3-(4-methoxybenzyl)-2,3-dihydro-1H-indene(3)
[0043]
[0044] Add compound 1-iodo-2-(pent-4-ene-2- Base) a mixed solution of benzene (237mg, 0.82mmol) and PEPPSI (11.1mg, 0.0164mmol) in 3 mL THF, and the reaction solution was stirred at room temperature for 5 minutes. The reaction solution was saturated with NH 4 Cl solution was quenched, extracted with ethyl acetate, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, the organic phase was concentrated, and the crude product was separated by column chromatography (eluent: PE:EA=100: 1) to obtain compound 3 (82mg, 45%) as a colorless oil. 1 H NMR (CDCl 3 , 400MHz,ppm):δ=7.62-6.86(m,8H),5.81-5.06(m,1H),5.81-5.06(m,2H),4.49-4.38(m,1H),3.90(s,3H) ,3.17(s,3H),2.50- 2.26(m,2H).
[0045] The transition metal salt PEPPSI ([1,3-bis(2,6-diisopropylphenyl) imidazol-2-yl subunit] (3-chloropyridyl) palladium dichloride (II) in the catalytic system of this...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com