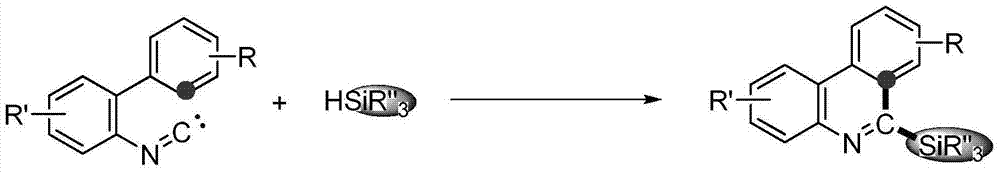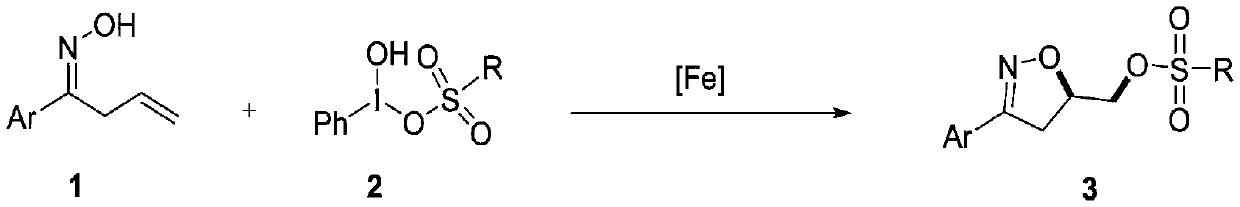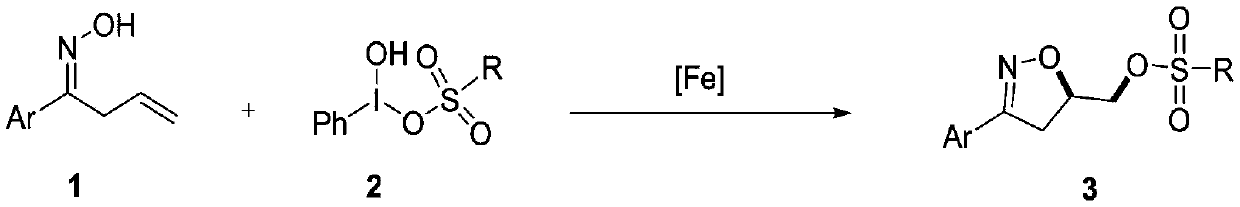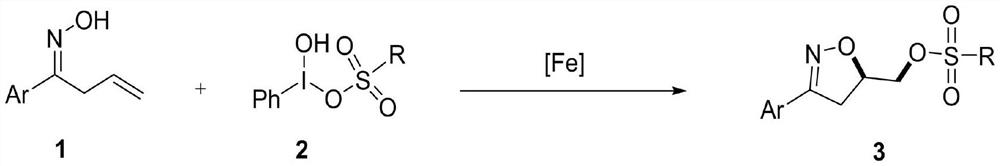Patents
Literature
33 results about "Radical cyclization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
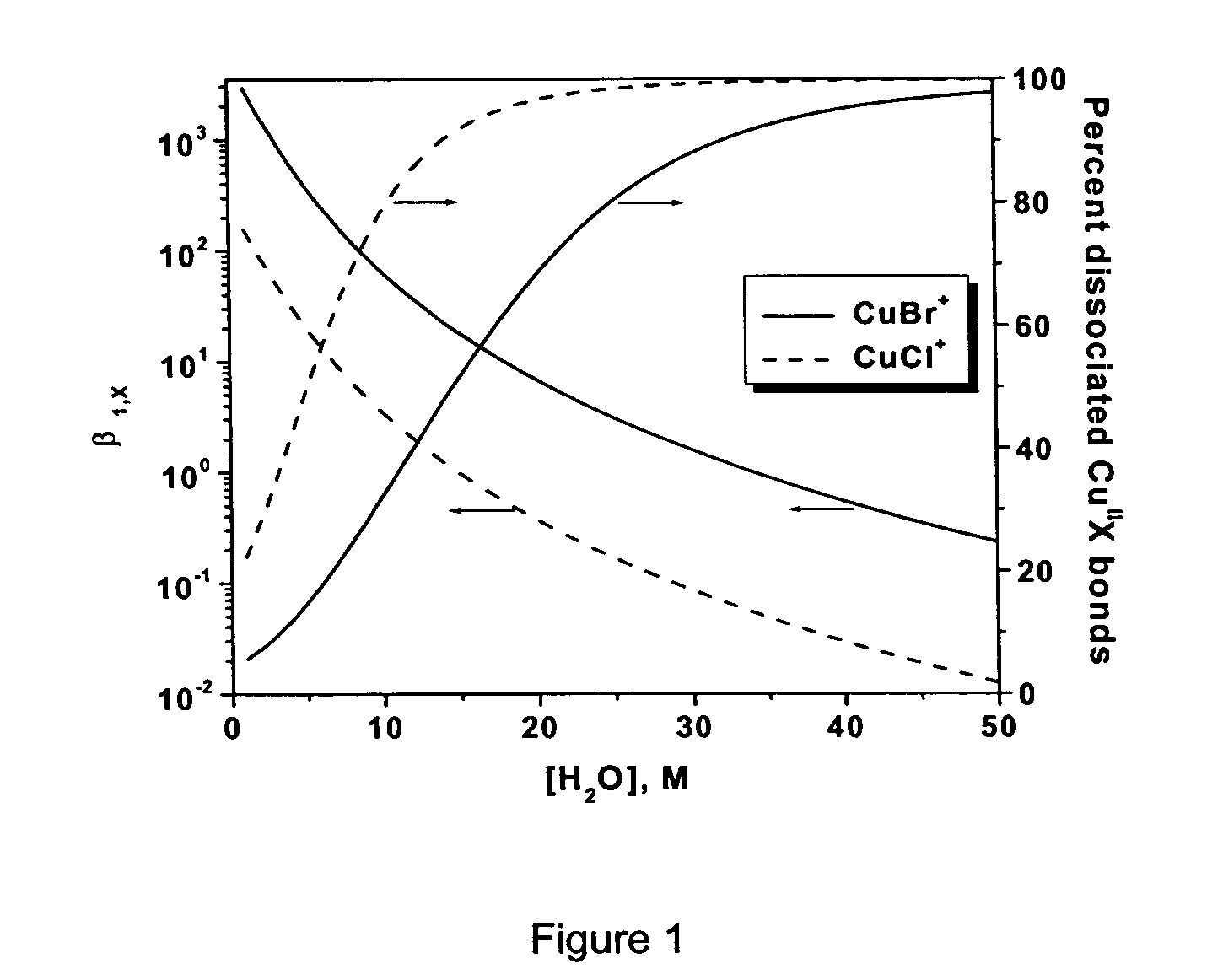
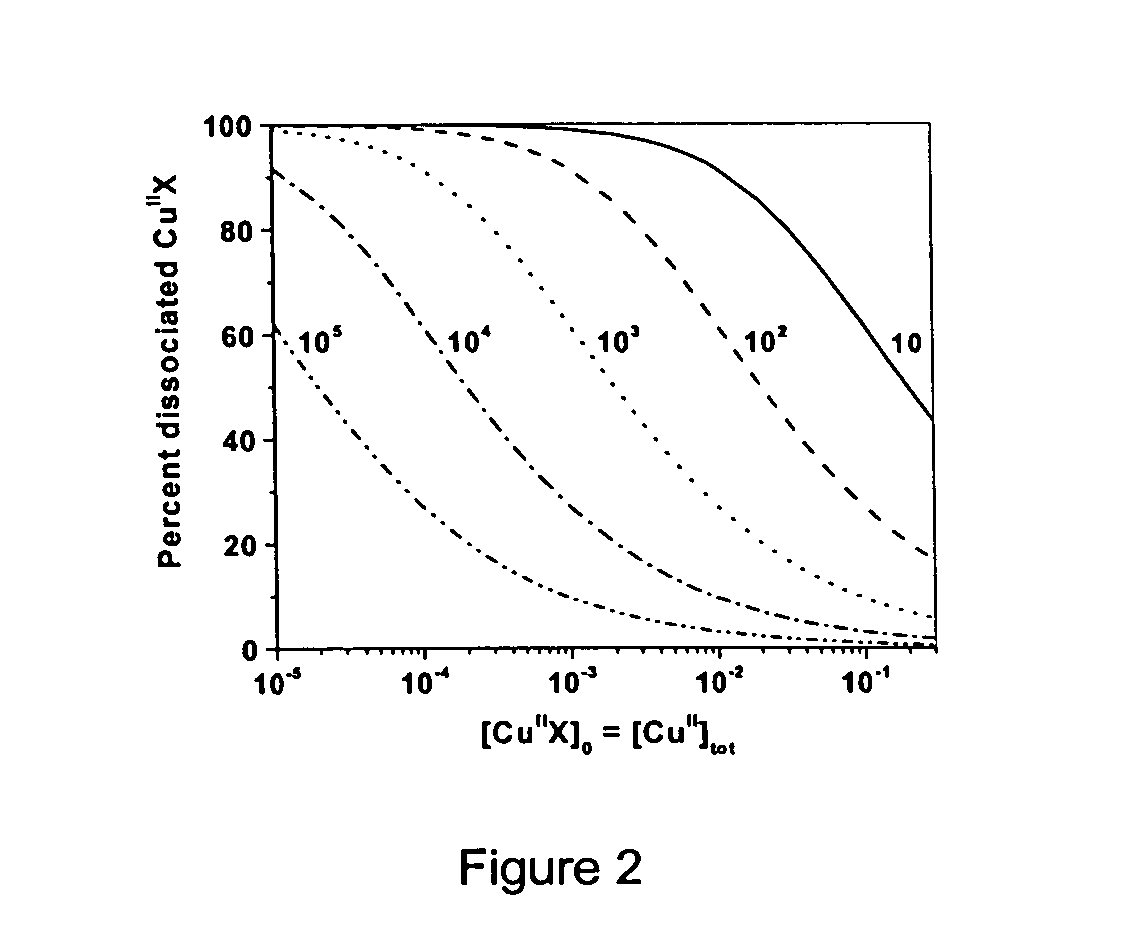
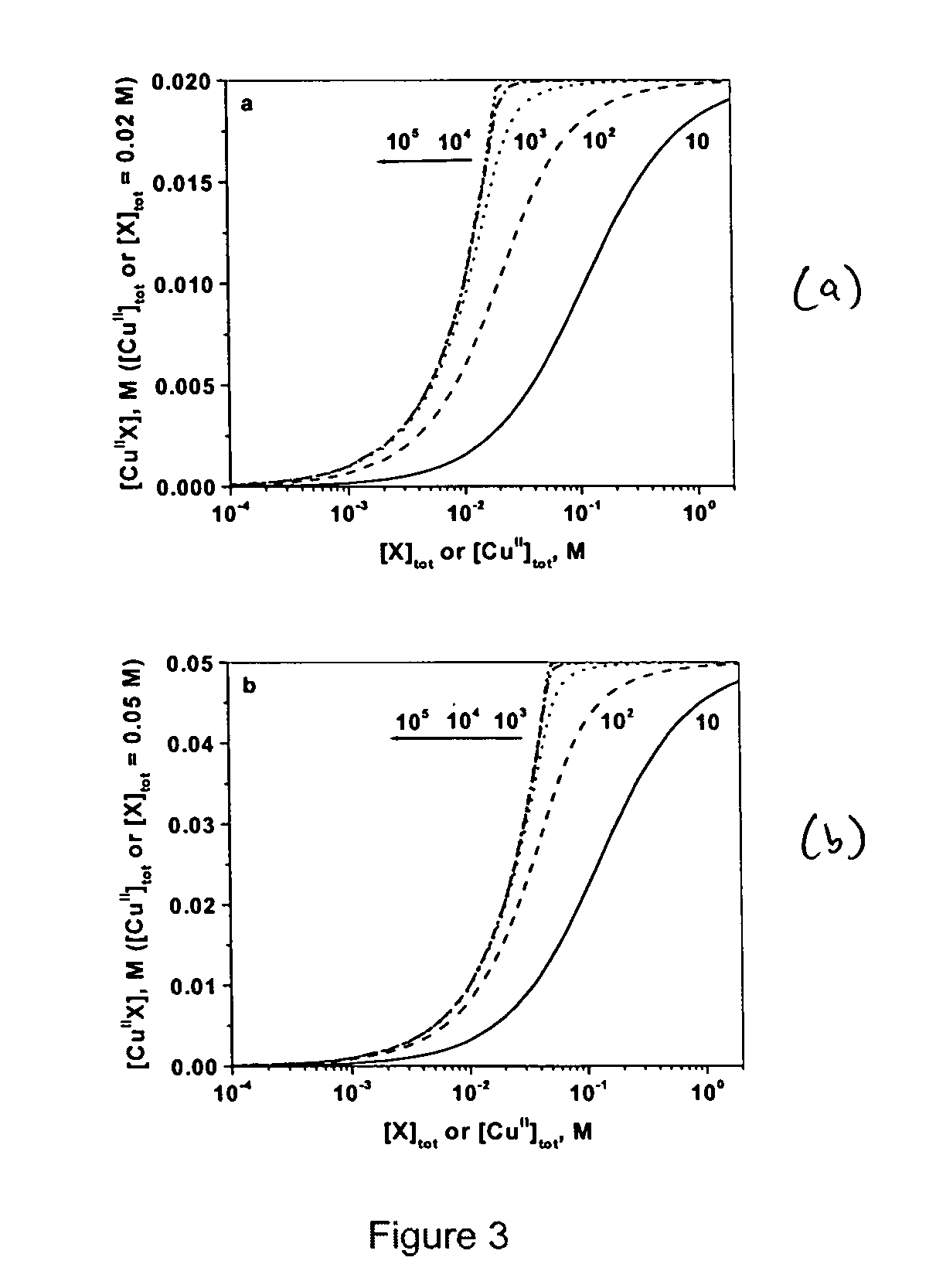
Radical cyclization reactions are organic chemical transformations that yield cyclic products through radical intermediates. They usually proceed in three basic steps: selective radical generation, radical cyclization, and conversion of the cyclized radical to product.
Stabilization of transition metal complexes for catalysis in diverse environments
This present invention is directed towards the identification or design, preparation, and use of suitable transition metal complexes for use as catalysts. The transition metal complexes may comprise heterodonor ligands. The present invention is also directed toward a method of determining the suitability of a transition metal complex for use in a catalytic reaction, such as, but not limited to, atom transfer radical polymerization (“ATRP”), atom transfer radical addition (“ATRA”), atom transfer radical cyclization (“ATRC”), and other catalytic redox reactions. The method assists in the approximate determination of the fundamental properties of the transition metal complex in a reaction media, such as, but not limited to, solubility, redox potential, stability towards acidic, basic, or ionic species, conditional radically transferable atom phylicity, and propensity toward disproportionation and therefore, the suitability of the complex to be used as a catalyst in the reaction media. The method provides a basis for prediction and evaluation of the properties of a transition metal complex for a particular selective catalytic reaction in a broad range of reaction environments. An understanding of the principles of the disclosed method allows a transition metal complex to be tuned to specific reaction medium by selecting a transition metal complex and ligand combination having the desired qualities.
Owner:CARNEGIE MELLON UNIV
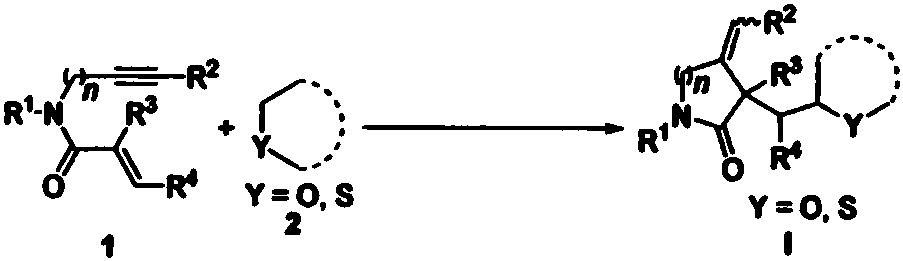
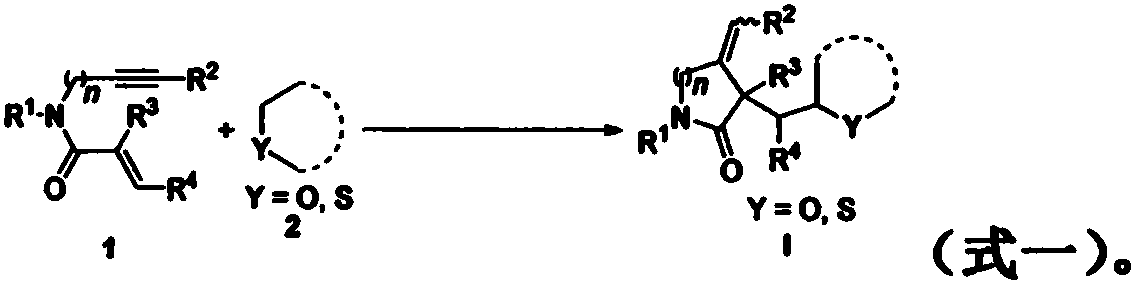
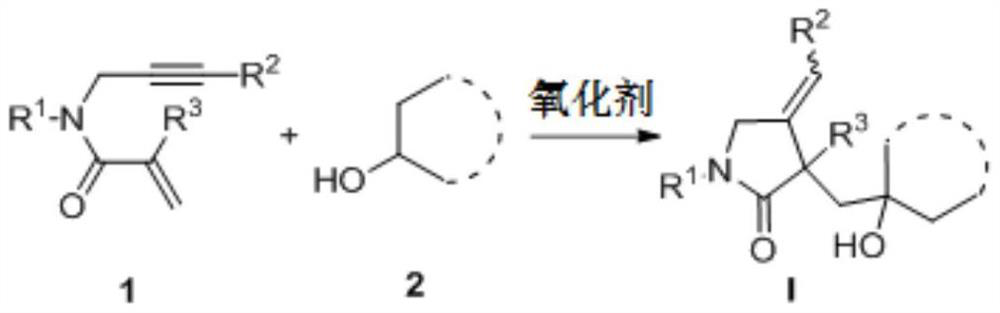
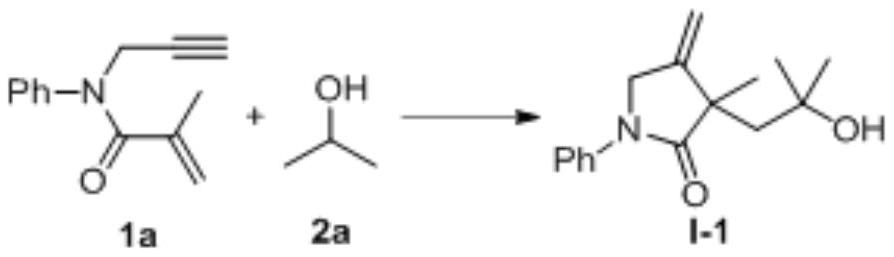
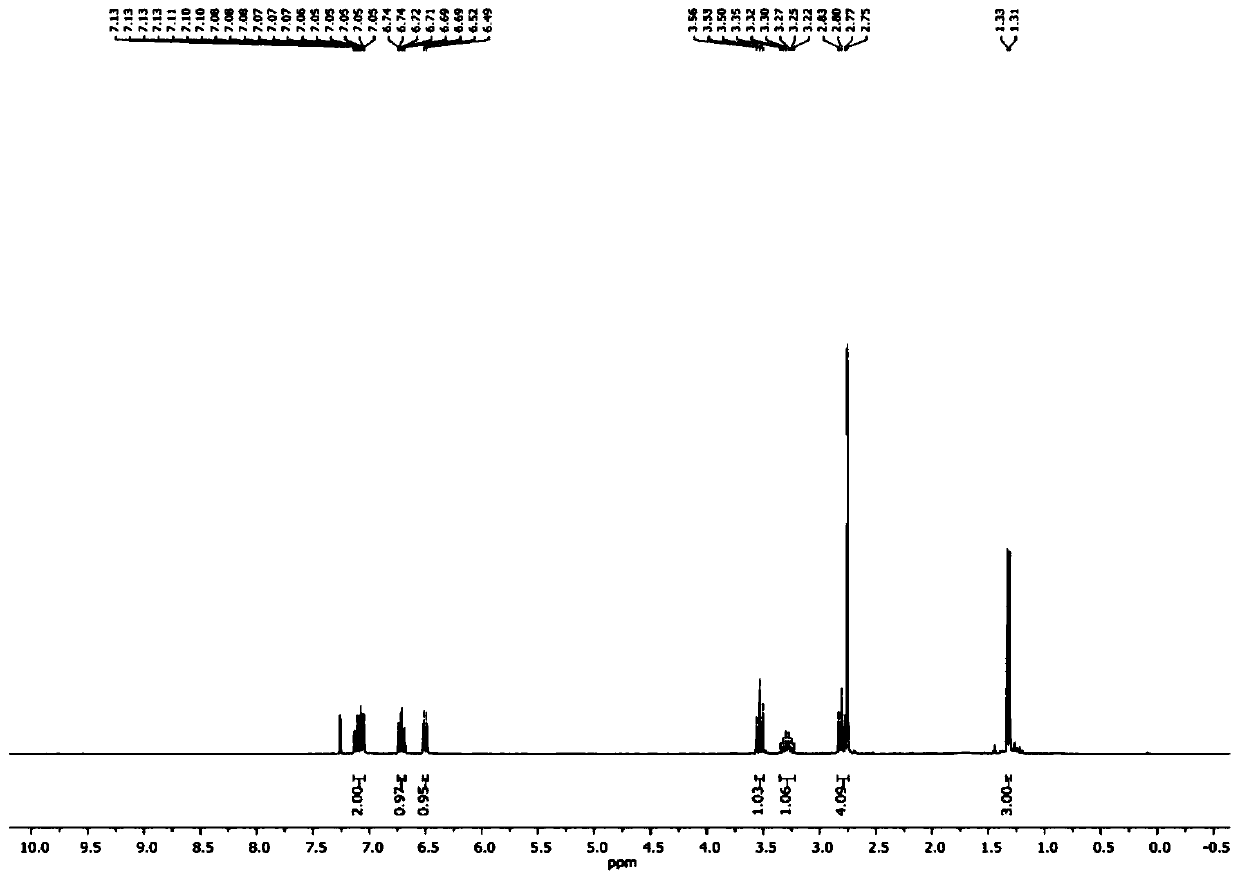
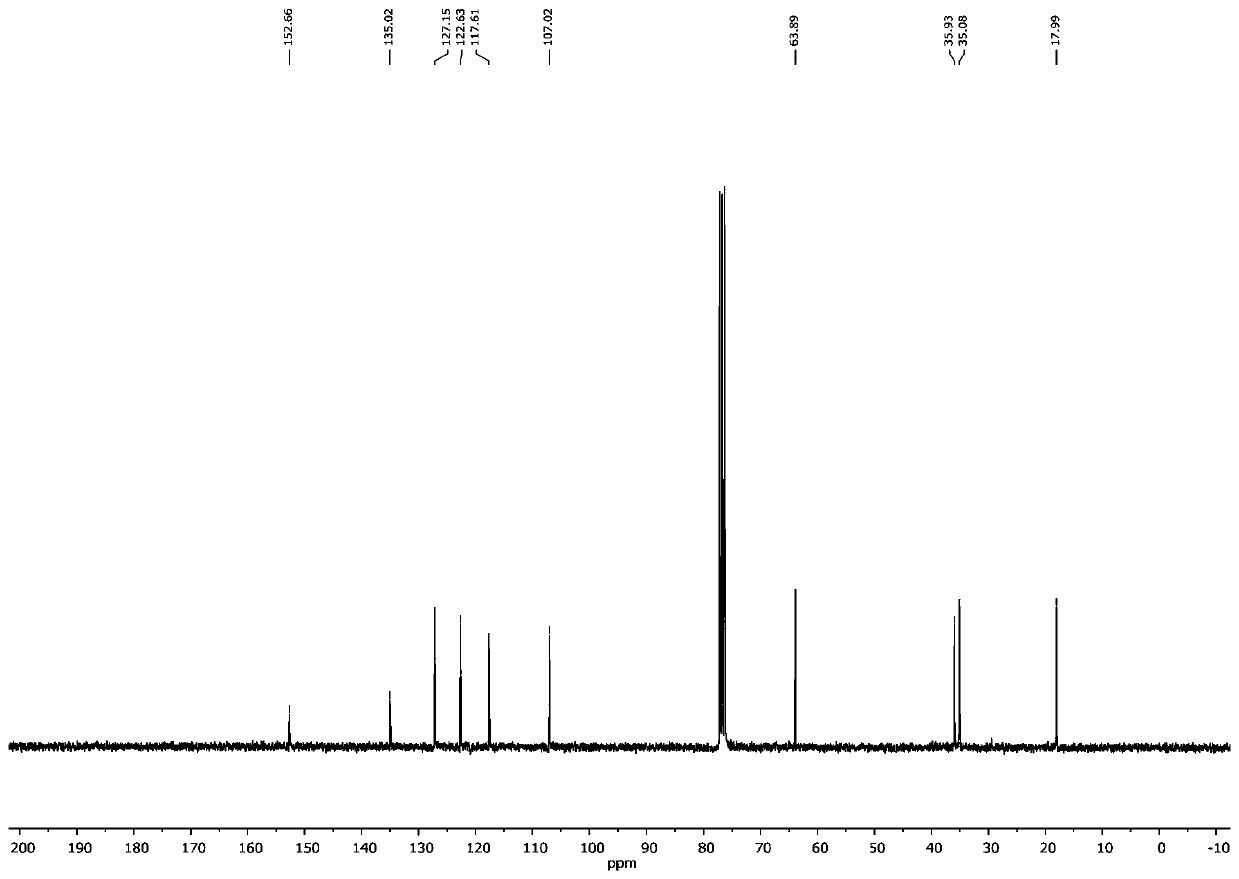
1,6-eneyne compound and alcohol compound based free radical cyclization reaction method
InactiveCN110054578ASuitable for industrial productionNo heavy metal residueOrganic chemistryEnvironmental resistanceAir atmosphere
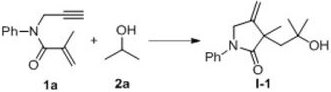
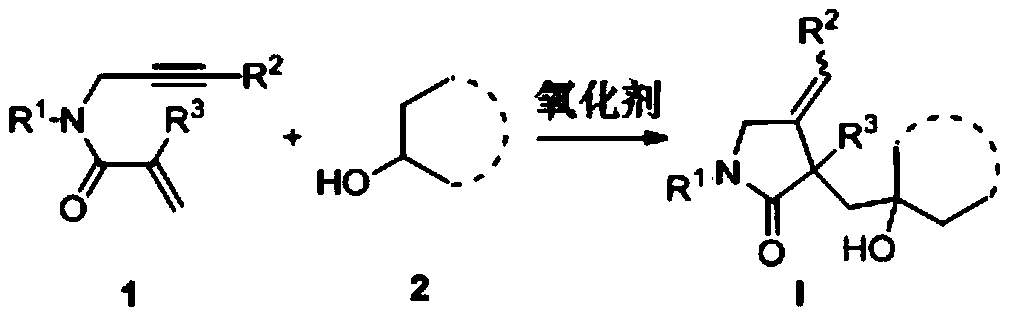
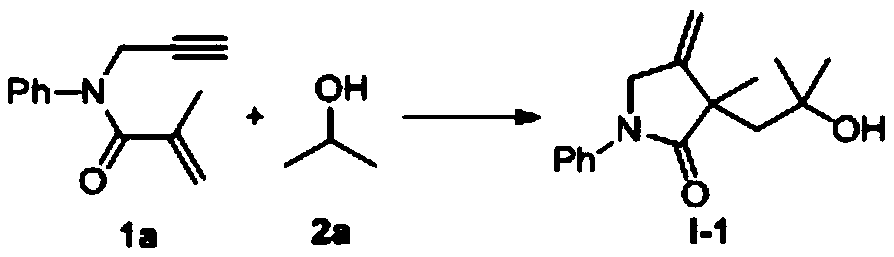
The invention belongs to the technical field of organic synthesis and discloses a 1,6-eneyne compound and alcohol compound based free radical cyclization reaction method. The method includes steps: taking an alcohol compound as a starting raw material, subjecting to cyclization reaction with a 1,6-eneyne compound under the action of an oxidant, and after complete reaction, performing aftertreatment to obtain a cyclization product namely a 2-pyrrolidone compound. The method has advantages that raw materials are cheap, easy to acquire, extensive in source, stable and low in toxicity, consumptionof any transition metal catalysts and ligands is avoided, the reaction can be carried out in an air atmosphere and is mild in condition and easy in operation, the product is free of heavy metal residues, and the method has advantages of extensive reaction substrate applicable range, simple, efficient, economical and green and is suitable for industrial production. In addition, an economical, practical, green and environmental-friendly novel idea is provided for alcohol free radical cyclization, and an application range is expanded.
Owner:YANGTZE NORMAL UNIVERSITY
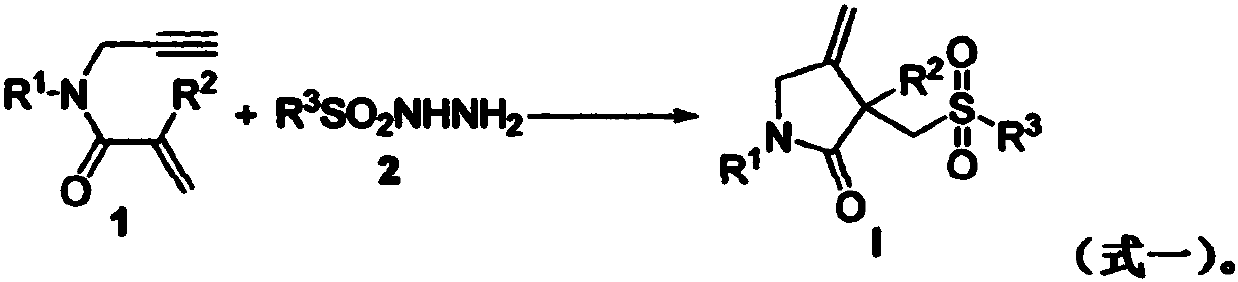
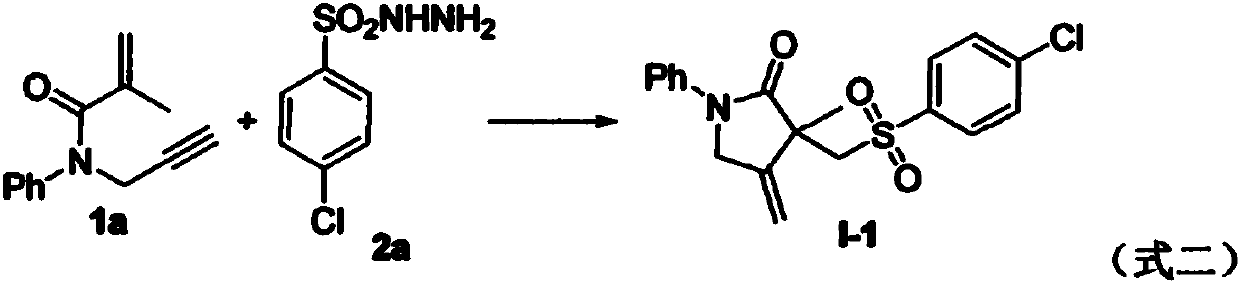
Free radical cyclization reaction method for 1,6-eneyne compounds and sulfonyl hydrazide compounds
ActiveCN110590637AMild reaction conditionsSuitable for industrial productionOrganic cyclisationAir atmosphereRegioselectivity
The invention relates to a high-regioselectivity free radical cyclization reaction method for 1,6-eneyne compounds and sulfonyl hydrazide compounds under mild conditions. The method comprises the following steps: adding one 1,6-eneyne compound, one sulfonyl hydrazide compound, a catalyst, an oxidant and a solvent into a Schlenk reaction flask, and performing a reaction under stirring under a certain temperature and air atmosphere condition to obtain one corresponding sulfone-containing cyclization product.
Owner:NINGBO UNIV
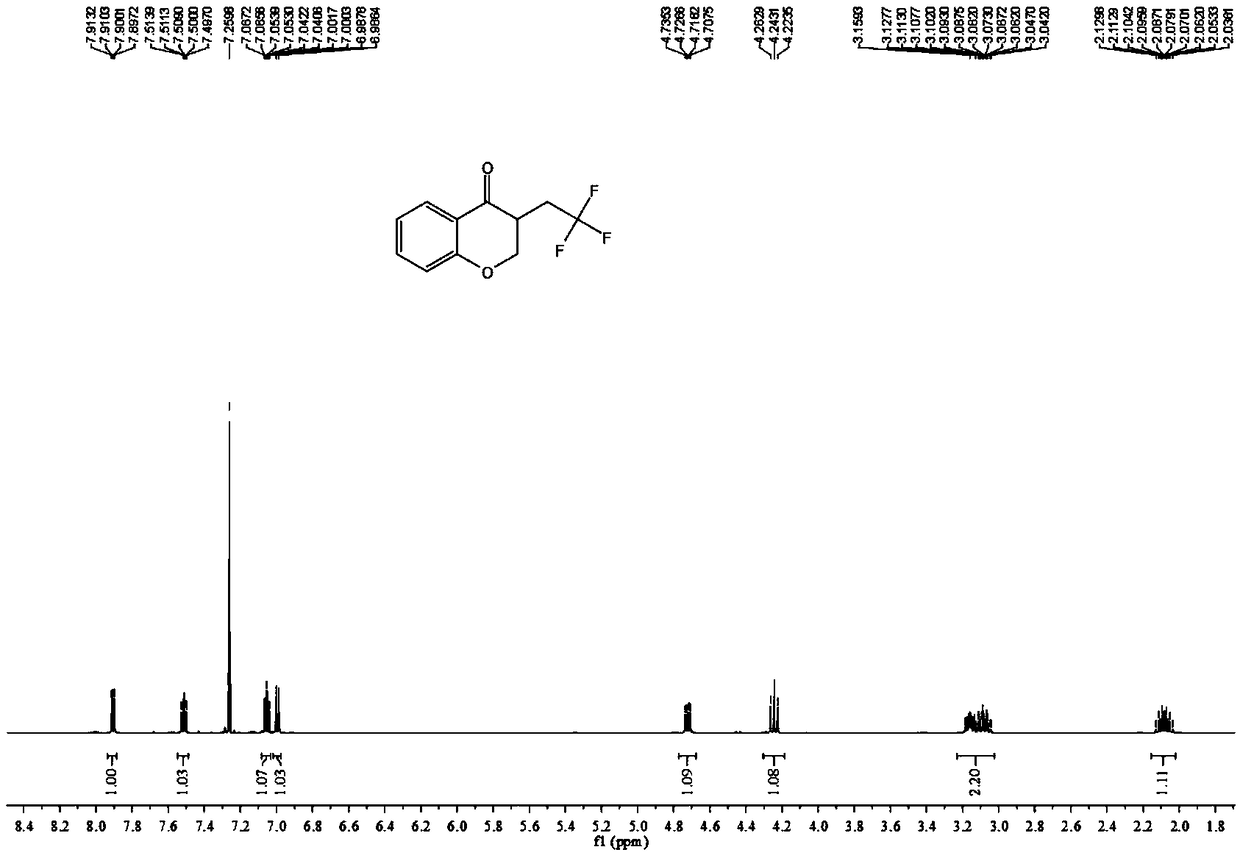
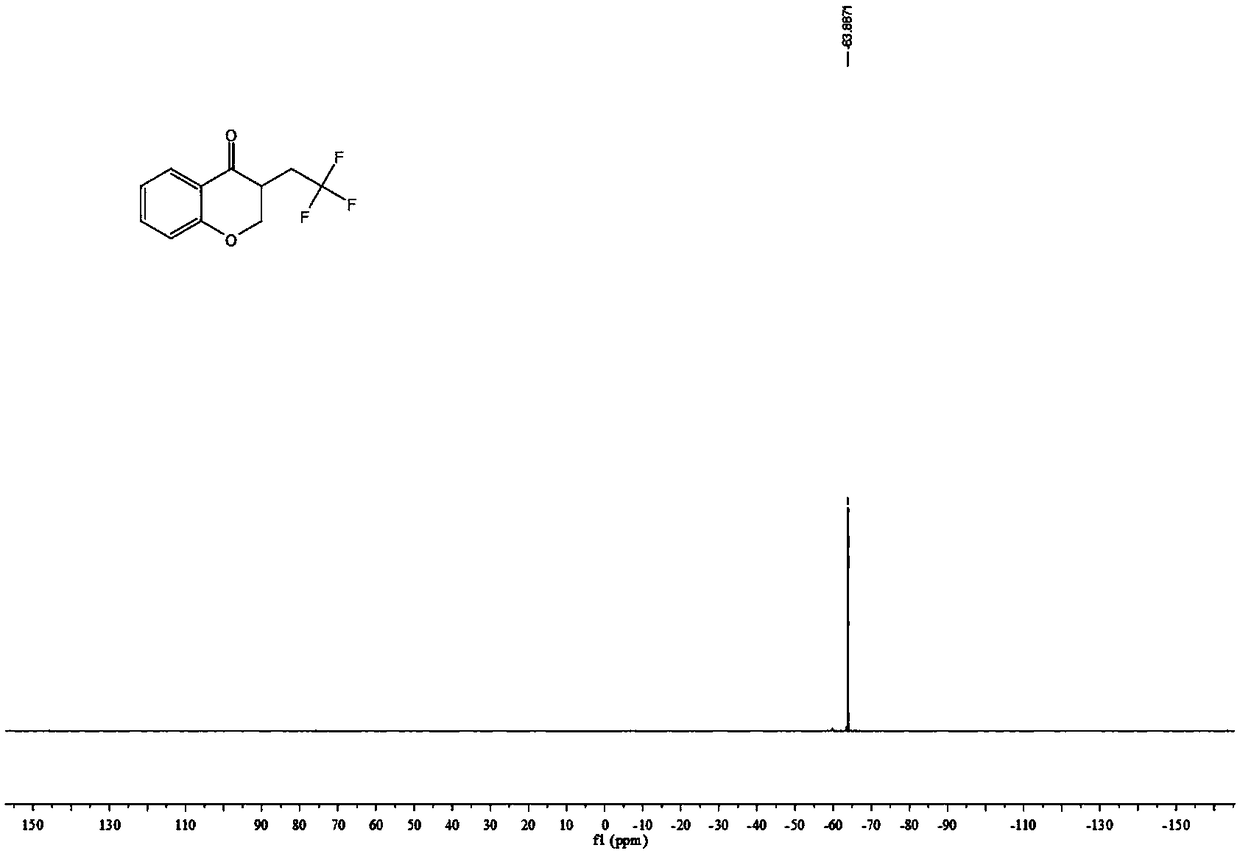
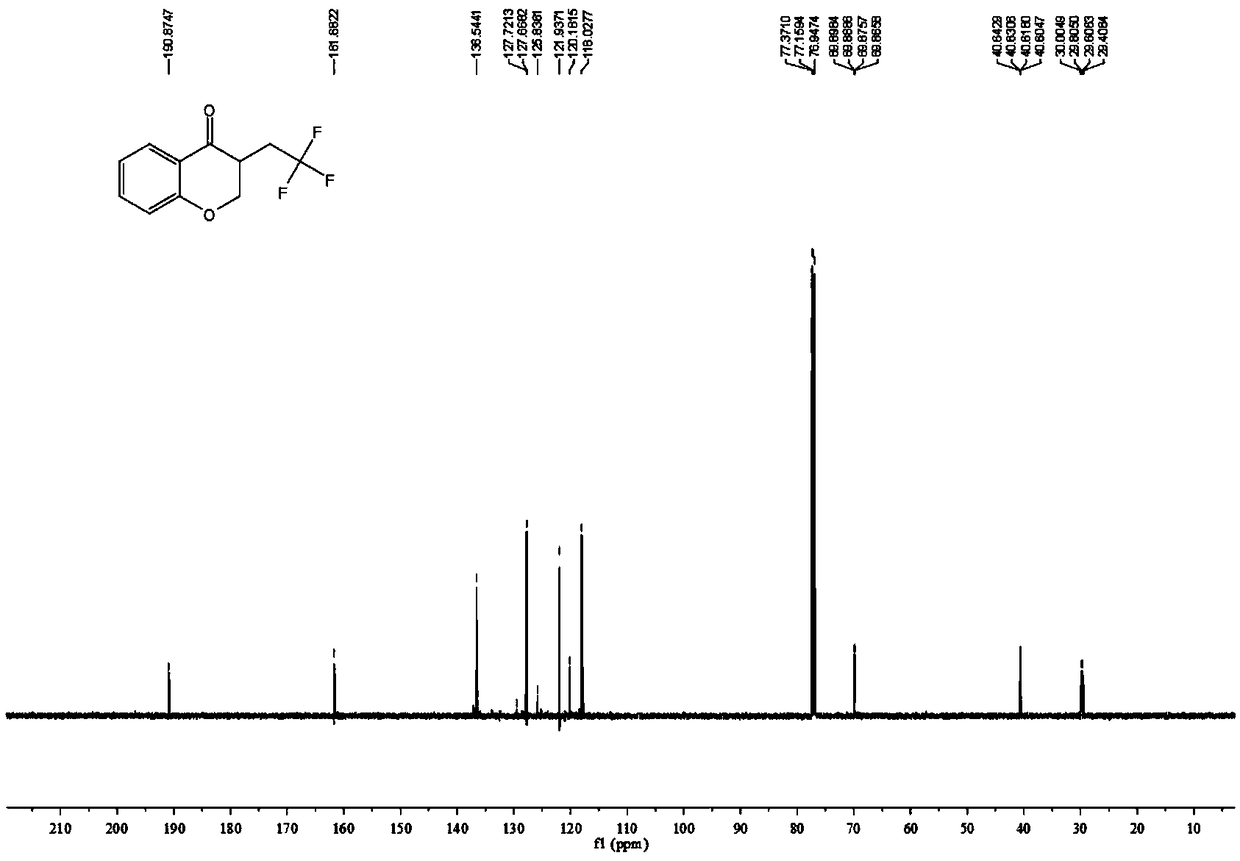
4-Chromanone compound containing trifluoromethyl and preparation method of 4-Chromanone compound
ActiveCN109400564AMild reaction conditionsEasy to operateOrganic chemistry4-chromanoneOxidizing agent
The invention provides a 4-Chromanone target compound (III) containing trifluoromethyl and a preparation method of the 4-Chromanone target compound (III), wherein the 4-Chromanone target compound (III) is obtained in the modes that an allylsalicylaldehyde (I) compound serves as a reaction starting raw material, sodium triflate (II) serves as a trifluoromethyl source, persulfate serves as an oxidant, and the trifluoromethyl and allylsalicylaldehyde generate free radical addition, free radical cyclization and an oxidation reaction. The reaction condition involved in the method is mild, operationis easy, product diversity is achieved, and gram-scale production can be achieved.
Owner:XINYANG NORMAL UNIVERSITY
Free radical cyclization reaction method of 1,6-eneyne compound and ketone compound
The invention relates to a method for implementing a double cyclization reaction of a 1,6-eneyne compound and a ketone compound by two-time ketone alpha-C(sp<3>)-H bond oxidation in a catalyst-free and alkali-free system. The method includes adding the 1,6-eneyne compound, the ketone compound and an oxidant into a Schlenk reaction flask, and stirring for reaction under a certain temperature and air atmosphere conditions to obtain a double cyclization product.
Owner:NINGBO UNIV
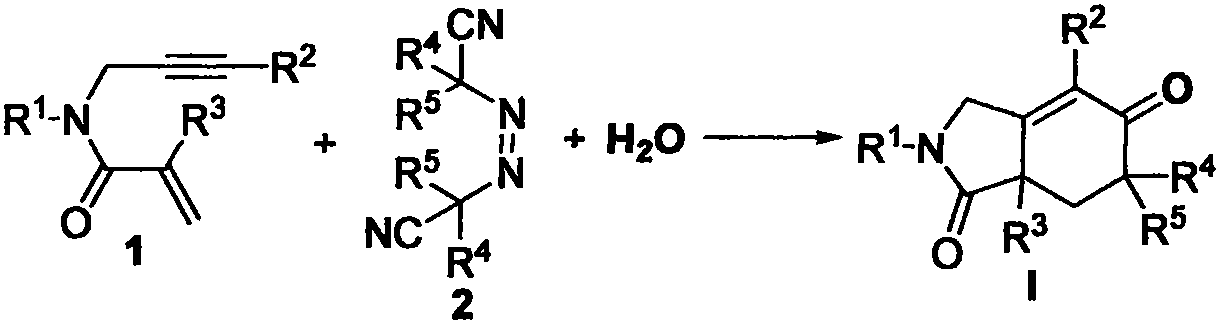
Free radical cyclization reaction method of 1, 6-enyne compound and azoalkyl nitrile
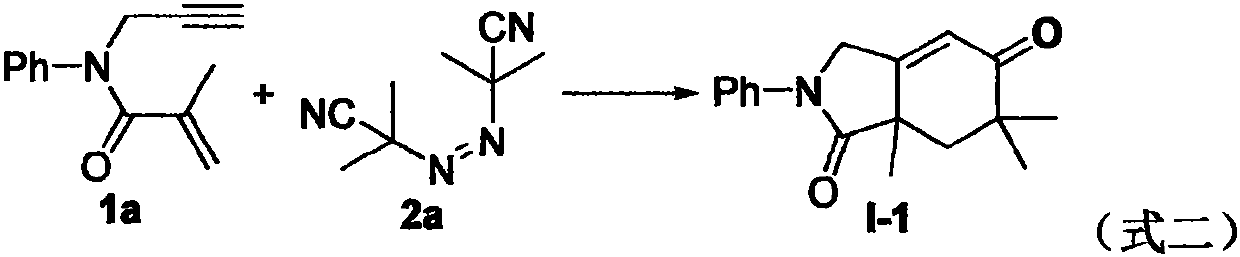
The invention relates to a regioselective free radical cyclization reaction method of a 1, 6-enyne compound and azoalkyl nitrile under a mild condition. According to the method, the 1, 6-enyne compound, an azoalkyl nitrile compound, a catalyst, alkali and a solvent are added into a Schlenk reaction bottle for stirring reaction under certain temperature and air atmosphere conditions, thus obtaininga cyclization product.
Owner:NINGBO UNIV
Free radical cyclization reaction method initiated by unactivated alkane C (sp3)-H functionalization
The invention relates to a method for preparing a benzimidazole isoquinolinone compound through free radical cyclization of 2-aryl benzimidazole initiated by C (sp3)-H bond functionalization of a cycloalkane or toluene compound in a water-phase metal-free system. The method comprises the following steps: adding a cycloalkane or toluene compound, a 2-aryl benzimidazole compound, an oxidant, a phase transfer agent and a solvent into a Schlenk reaction flask, and stirring and reacting at a certain temperature in an air atmosphere to obtain the benzimidazole isoquinolinone compound.
Owner:NINGBO UNIV
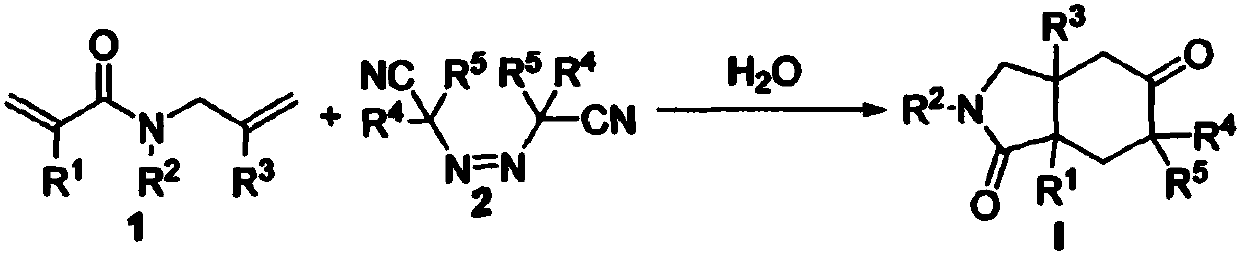
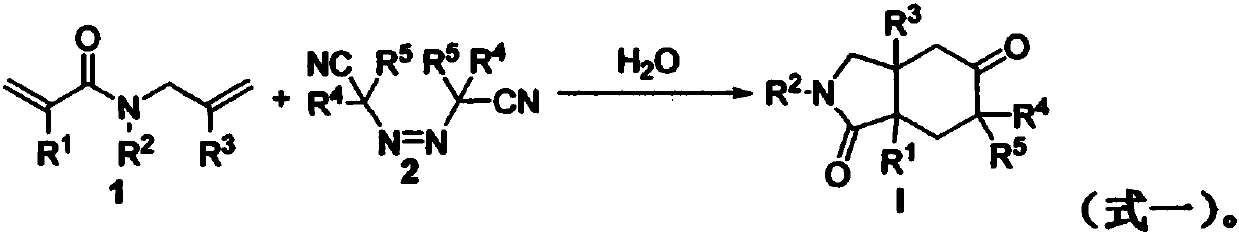
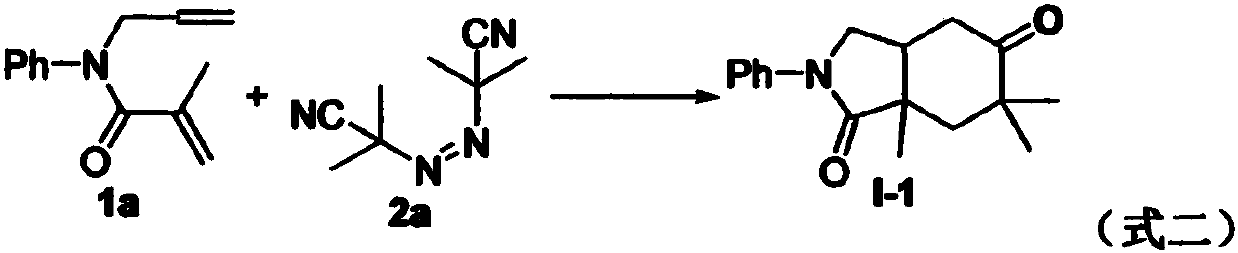
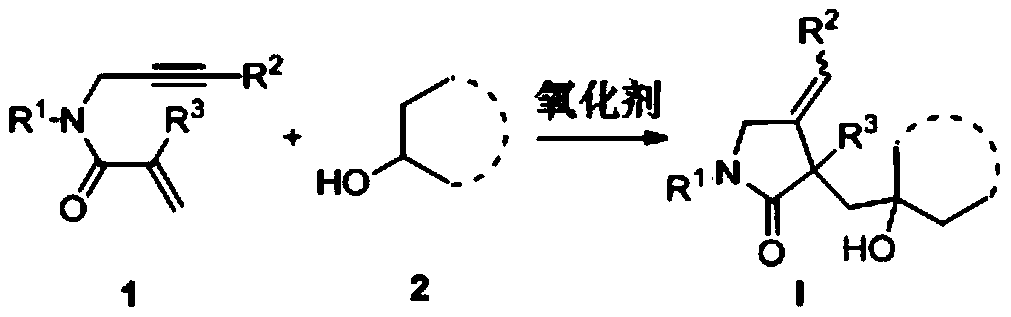
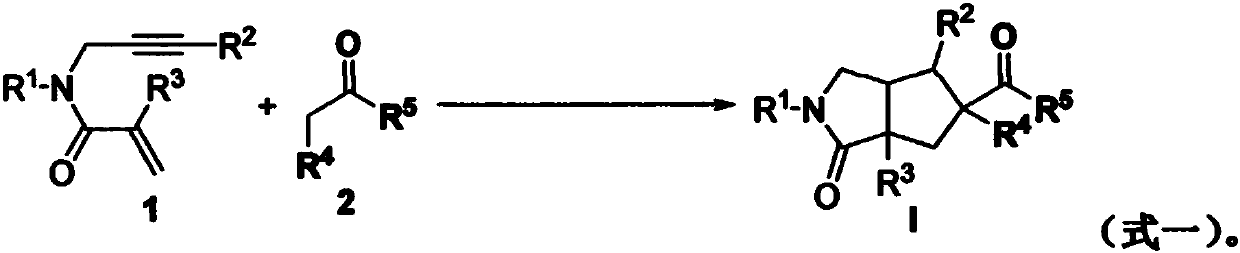
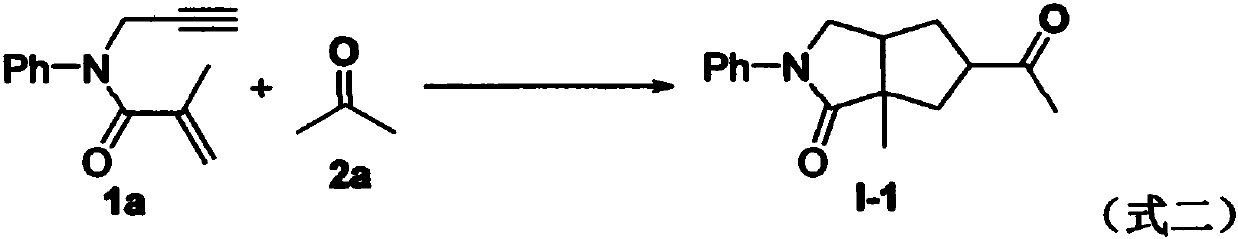
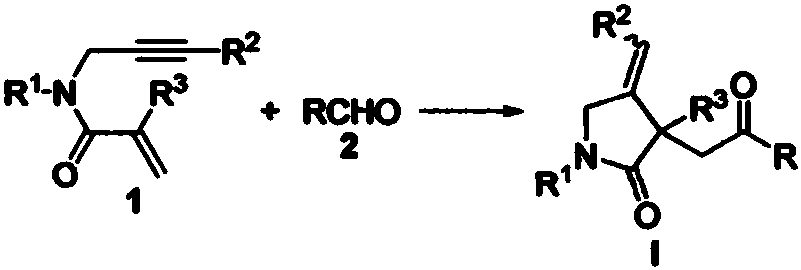
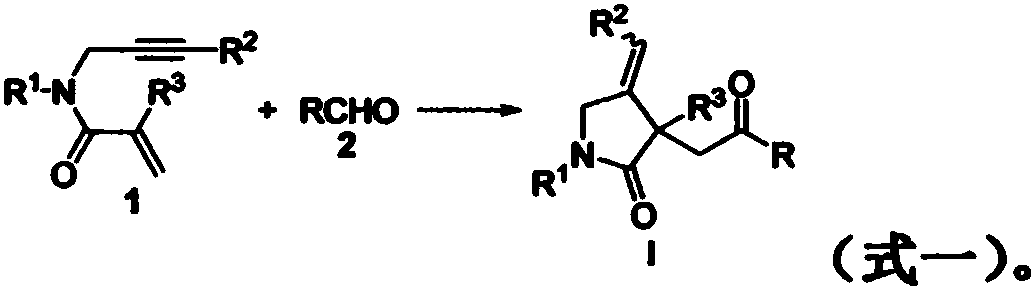
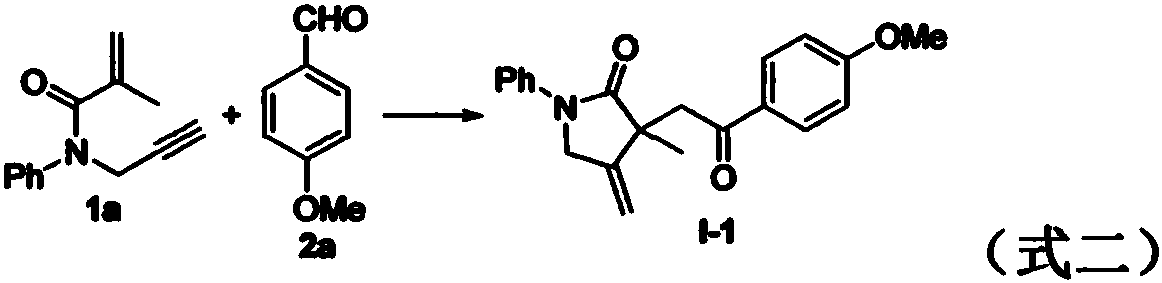
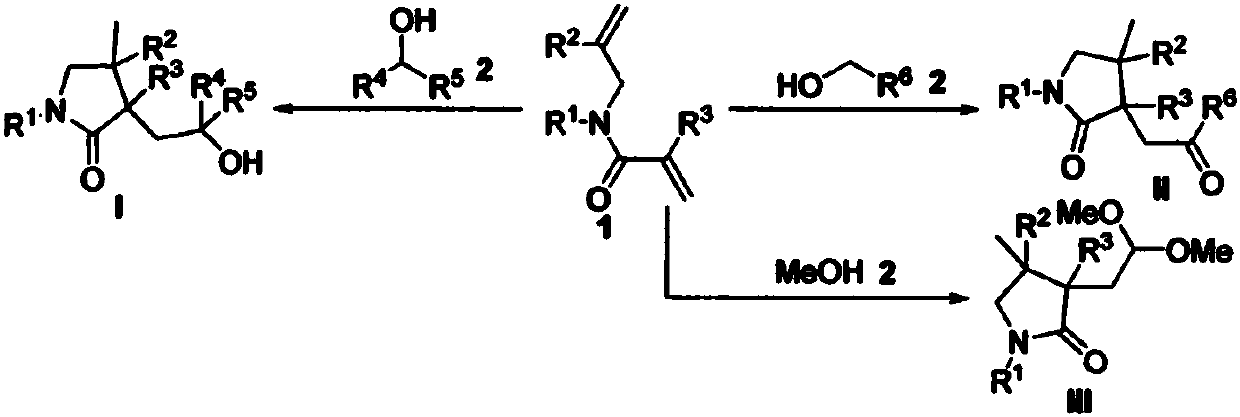
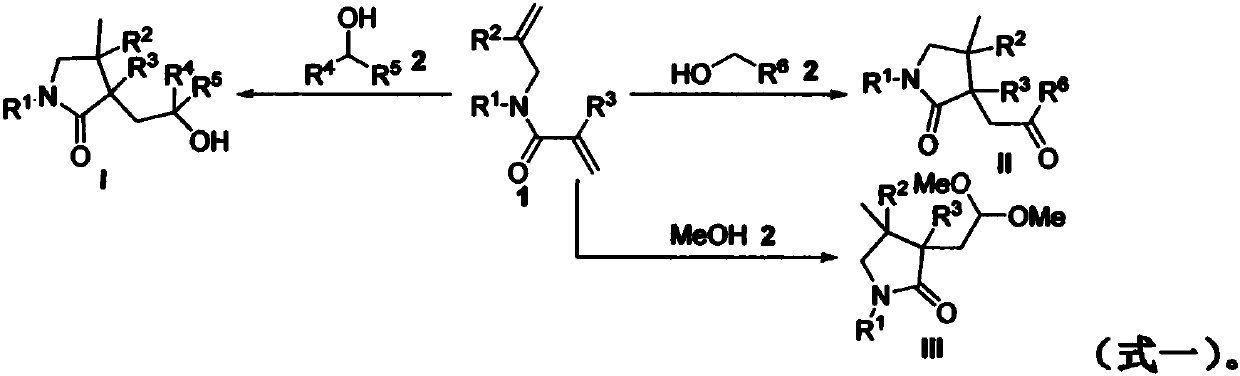
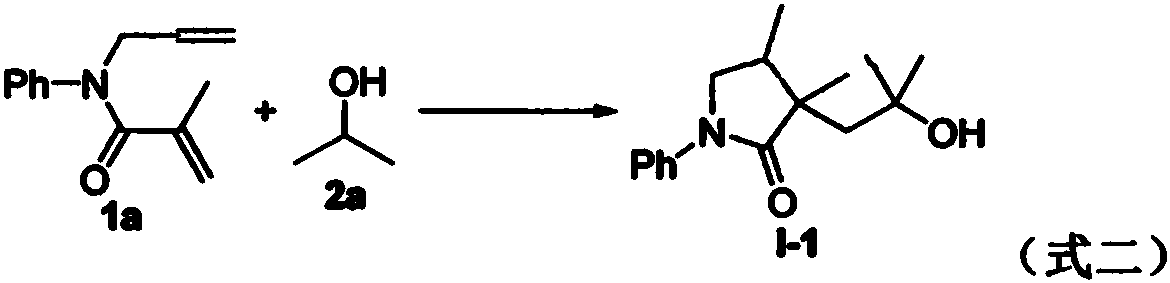
Preparation method of 2-pyrrolidone derivative
The invention relates to a preparation method of a 2-pyrrolidone derivative. According to the method, 1,6-eneyne compounds, carbonyl compounds, oxidizing agents and organic solvent are added into a Schlenk reaction flask, the mixture is stirred on the conditions of certain temperature and the air atmosphere to carry out free radical cyclization reaction.
Owner:NINGBO UNIV
Free radical reaction method of 1, 6-diene and alcohol in additive-free system
ActiveCN111233732ASuitable for industrial productionOrganic chemistryChemical recyclingAir atmosphereAlcohol
The invention discloses a free radical reaction method of 1, 6-diene and alcohol in an additive-free system, and relates to a regioselective free radical cyclization reaction method of a 1, 6-diene compound and an alcohol compound in a catalyst-free and alkali-free system under mild conditions. The method comprises the following steps: adding a 1, 6-diene compound, an alcohol compound and an oxidizing agent into a Schlenk reaction flask, and carrying out a stirring reaction process at a certain temperature under an air atmosphere condition to obtain a cyclized product.
Owner:NINGBO UNIV
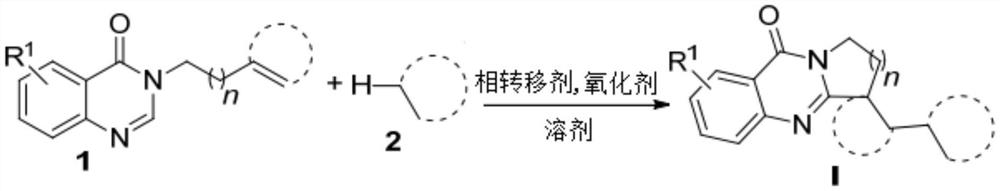
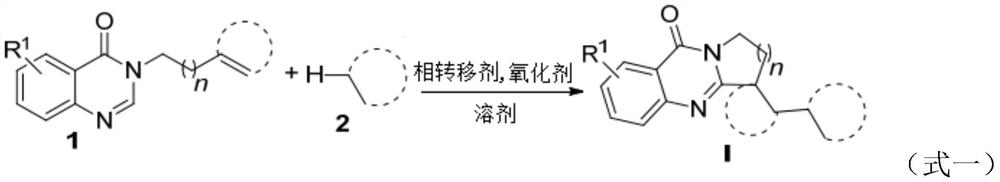
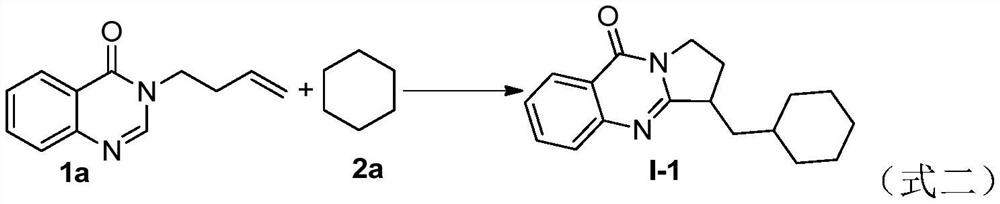
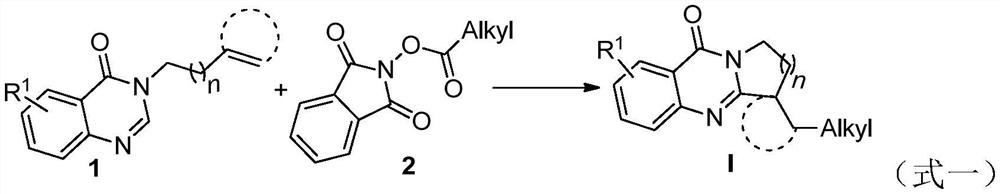
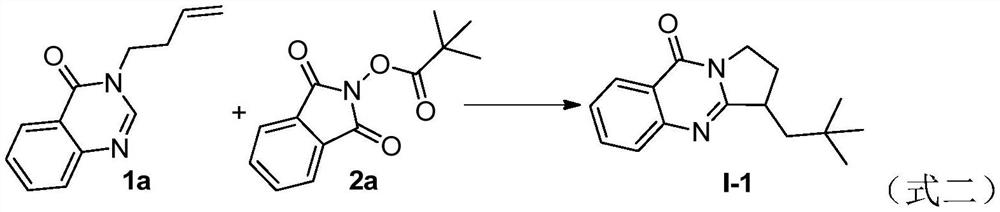
Benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone and derivative and preparation method thereof
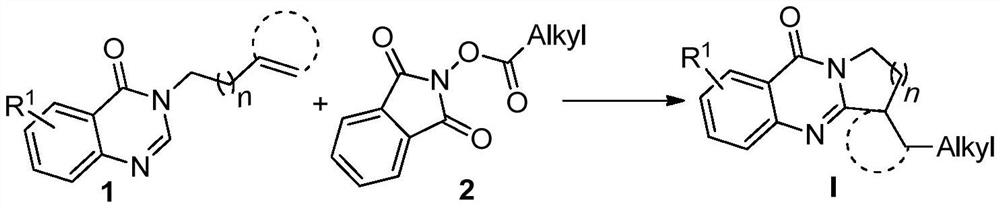
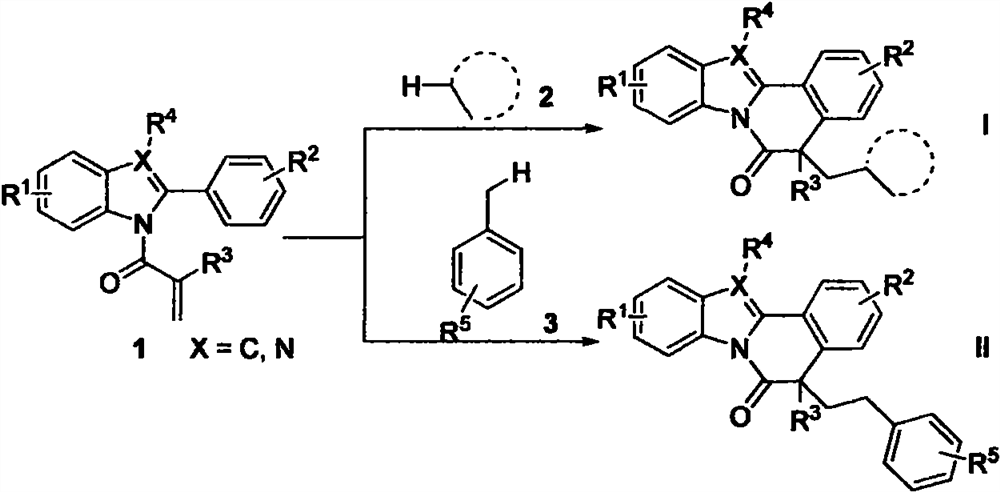
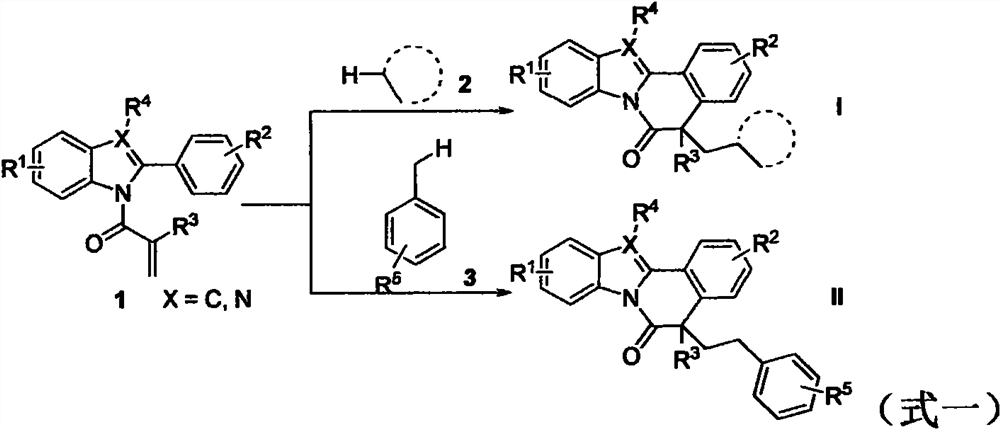
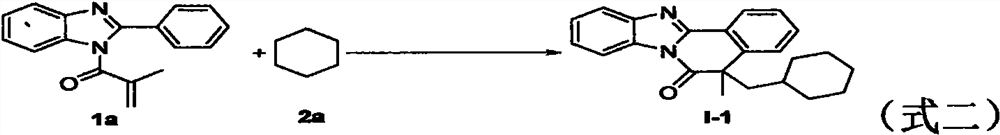
The invention relates to benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone as well as a derivative and a preparation method thereof. The benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone has a structure as shown in a formula (I) defined in the description. According to the invention, a benzo [4, 5] imidazo [2, 1-a] isoquinoline tetracyclic framework is synthesized by a visible light driven cascade free radical cyclization reaction, alpha-carbonyl alkyl generated by a bromide can be subjected to intermolecular free radical addition on a C-C double bond, then an intramolecular C-C bond is formed, and a series of benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone is synthesized.
Owner:SHANGHAI UNIV
Free radical cyclization reaction method of 1,6-eneyne compound and ether compound
The invention relates to a regioselective free radical cyclization reaction method of a 1,6-eneyne compound and an ether compound in a transition-metal-free catalyst system. The method comprises the following steps: adding a 1,6-enyne compound, an ether compound, an alkali additive and an oxidizing agent into a Schlenk reaction bottle, and carrying out a stirring reaction at a certain temperatureunder an air atmosphere condition to obtain a cyclization product 2-pyrrolidone compound.
Owner:NINGBO UNIV
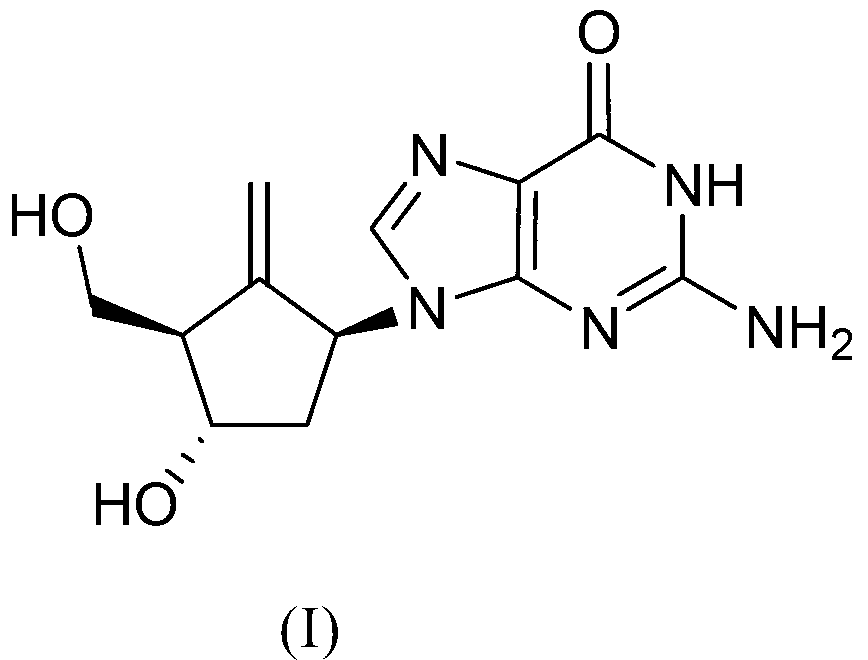
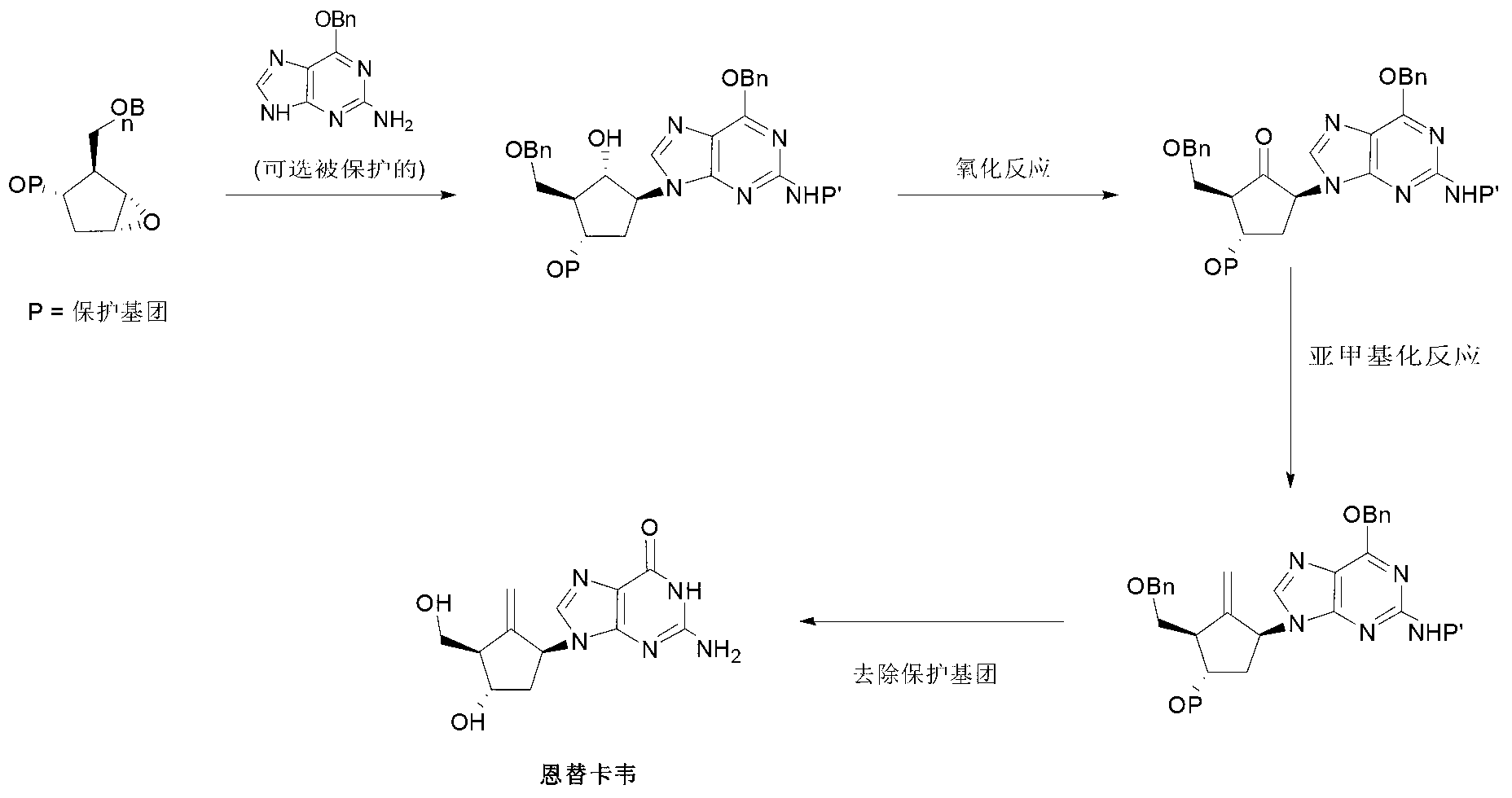
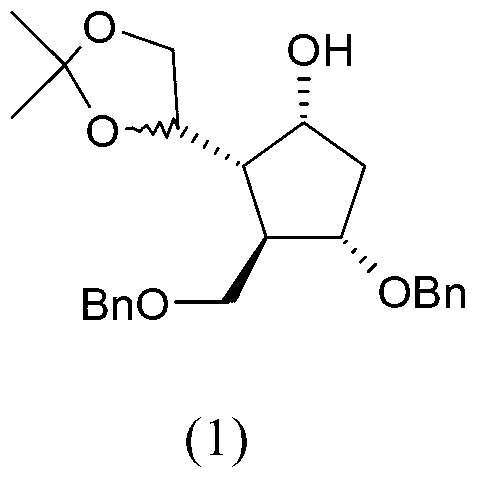
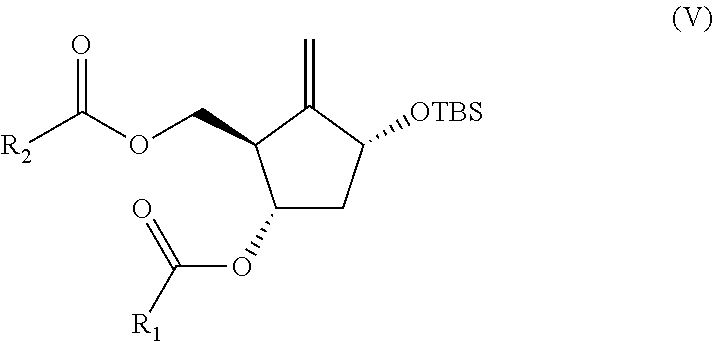
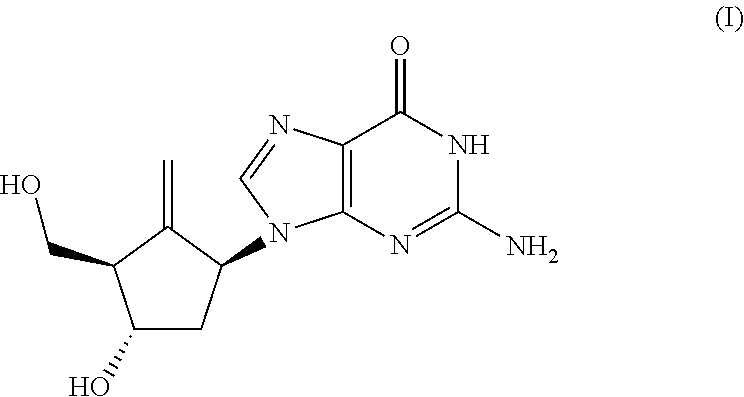
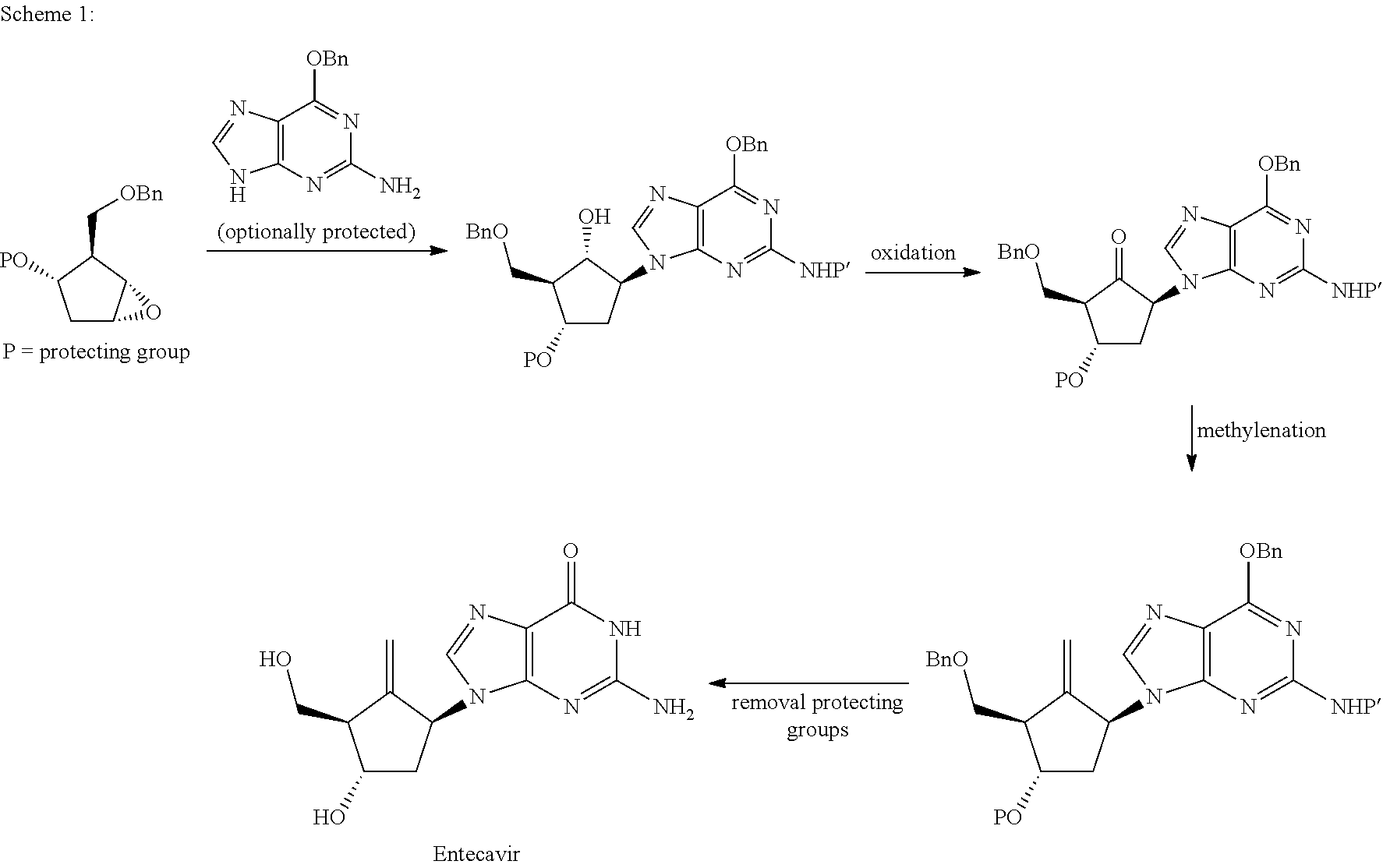
Preparation process of an antiviral drug (entecavir) and intermediates thereof
It comprises a preparation process of entecavir comprising: submitting a (1S, 3R)-3-(tert-butyldimethylsilyloxy)-1 -(oxiran-2-yl)pent-4-yn-1-ol (VIII) to a double esterification and to a radicalary cyclization, yielding a compound of formula (V), where either a compound of formula (VIII) is submitted to a first esterification reaction, then to a catalytic radicalary cyclization using titanocene dichloride as catalyst in the presence of Mn / 2,4,6-collidine HCI or Zn / 2,4,6-collidine / trimethylsilyl chloride, and finally to a second esterification reaction or, alternatively, the compound of formula (VIII) is submitted first to a catalytic radicalary cyclization, and then to an esterification reaction. Entecavir can be obtained by submitting compound (V) to a desilylation reaction to remove the TBS group and then to a Mitsunobu coupling with 2- amino-6-chloroguanine, followed by hydrolysis. It also relates to some new intermediates of the process.
Owner:ESTEVE QUIMICA
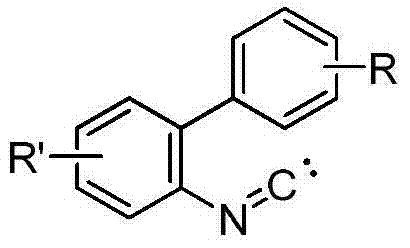
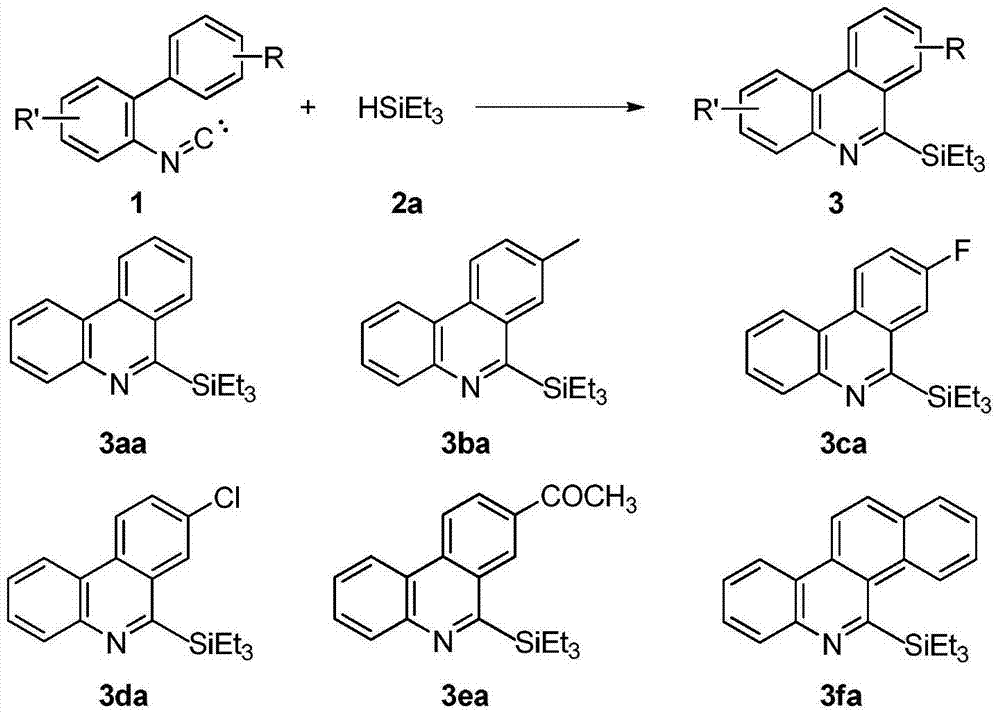
Method for synthesizing phenanthridine silane derivative
InactiveCN103936781AEasy to handleEasy to operateGroup 4/14 element organic compoundsSilanesCatalytic oxidation
The invention discloses a method for synthesizing a phenanthridine silane derivative, and relates to the fields of organic chemical engineering and fine chemical engineering. A small amount of organic solvent is added into 2-aryl aromatic isonitrile and silane under catalytic oxidation action of an oxidizing agent and a free radical initiator, and the mixture is subjected to cyclization reaction under the room temperature or heating condition to obtain the phenanthridine silane derivative. By using the method provided by the invention, the reaction time is 4-12 hours under the heating and stirring condition; the raw materials are reacted between the room temperature and 120 DEG C and are subjected to simple after-treatment so as to obtain the corresponding phenanthridine silane derivative with high yield of 68-93 percent. A novel aryl sp<2> silicon carbon bond formation method based on a free radical process is developed, two steps of cyclizing the free radicals and forming a silicon carbon bond are carried out to obtain a series of phenanthridine silane derivatives; therefore, the method is easy to carry out and is convenient for after-treatment.
Owner:CHANGZHOU UNIV
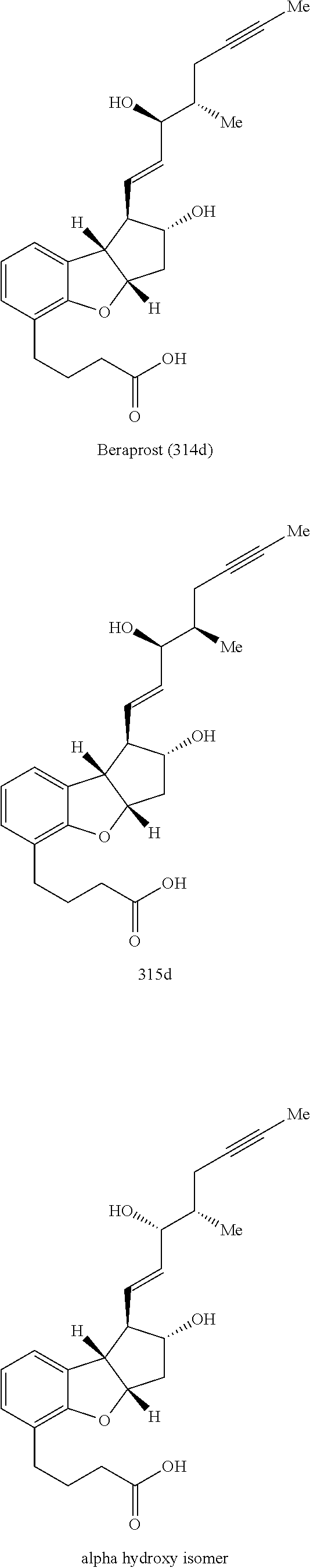
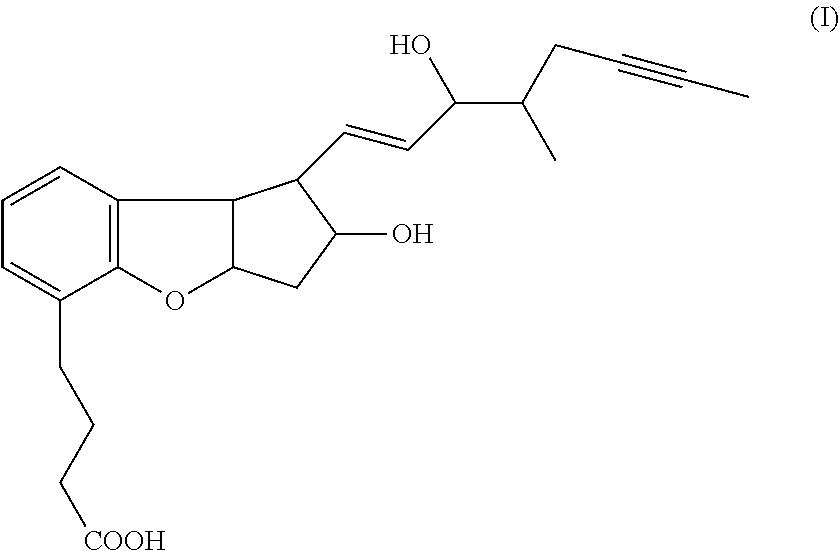
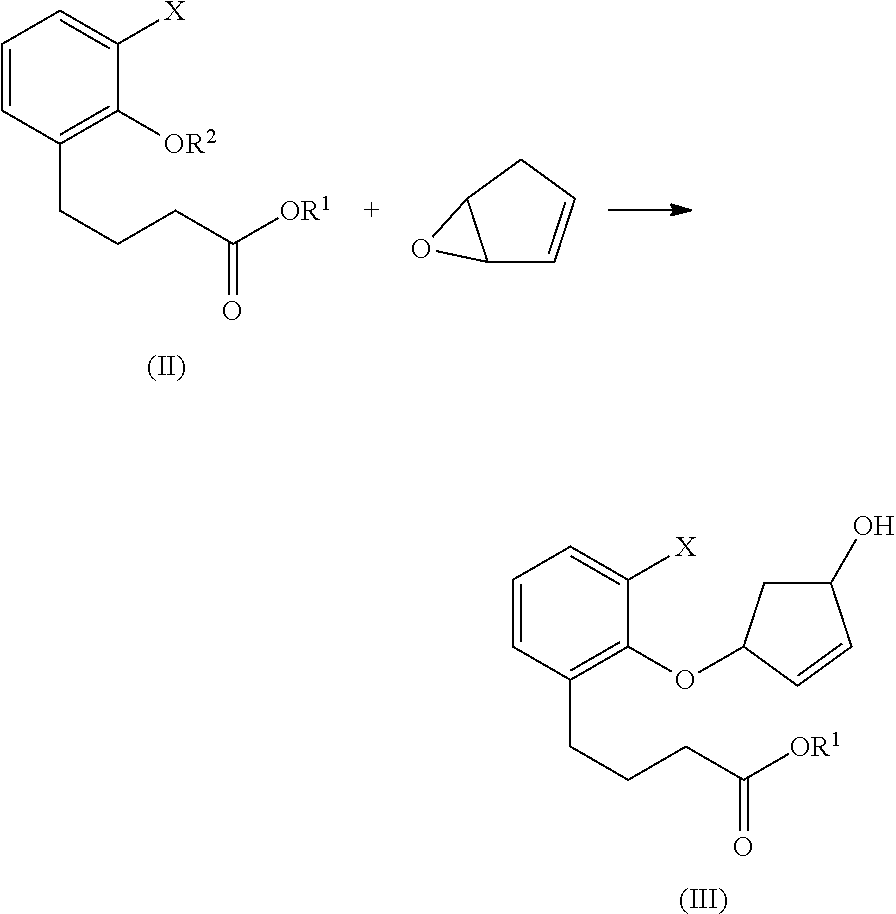
Methods for producing beraprost and its derivatives
ActiveUS10005753B2Organic active ingredientsOrganic compound preparationDrug compoundStereoselectivity
The present invention is directed to methods for preparing Beraprost and novel synthetic intermediates for Beraprost. In one aspect, a process is provided to produce a pharmaceutical compound represented by the general Formula (I) via a radical cyclization route. The process is completed in fewer steps than the known synthetic methods and may be conducted to prepare commercially useful quantities. In another aspect, synthetic methods are provided for producing Beraprost and its derivatives, which are stereoselective, efficient, scalable and economical. In another aspect, substantially isomerically pure compounds and intermediates are produced by the above processes.
Owner:LUNG BIOTECH PBC
Preparation process of an antiviral drug (entecavir) and intermediates thereof
InactiveUS20130296558A1Reduce formationLower reaction conditionsSilicon organic compoundsAntiviralsTrimethylsilyl chlorideAntiviral drug
It comprises a preparation process of entecavir comprising: submitting a (1S,3R)-3-(tert-butyldimethylsilyloxy)-1-(oxiran-2-yl)pent-4-yn-1-ol (VIII) to a double esterification and to a radicalary cyclization, yielding a compound of formula (V), where either a compound of formula (VIII) is submitted to a first esterification reaction, then to a catalytic radicalary cyclization using titanocene dichloride as catalyst in the presence of Mn / 2,4,6-collidine HCl or Zn / 2,4,6-collidine / trimethylsilyl chloride, and finally to a second esterification reaction or, alternatively, the compound of formula (VIII) is submitted first to a catalytic radicalary cyclization, and then to an esterification reaction. Entecavir can be obtained by submitting compound (V) to a desilylation reaction to remove the TBS group and then to a Mitsunobu coupling with 2-amino-6-chloroguanine, followed by hydrolysis. It also relates to some new intermediates of the process.
Owner:ESTEVE QUIMICA
Methods for producing beraprost and its derivatives
ActiveUS20170166545A1Few stepsOrganic active ingredientsOrganic compound preparationDrug compoundStereoselectivity
The present invention is directed to methods for preparing Beraprost and novel synthetic intermediates for Beraprost. In one aspect, a process is provided to produce a pharmaceutical compound represented by the general Formula (I) via a radical cyclization route. The process is completed in fewer steps than the known synthetic methods and may be conducted to prepare commercially useful quantities. In another aspect, synthetic methods are provided for producing Beraprost and its derivatives, which are stereoselective, efficient, scalable and economical. In another aspect, substantially isomerically pure compounds and intermediates are produced by the above processes.
Owner:LUNG BIOTECH PBC
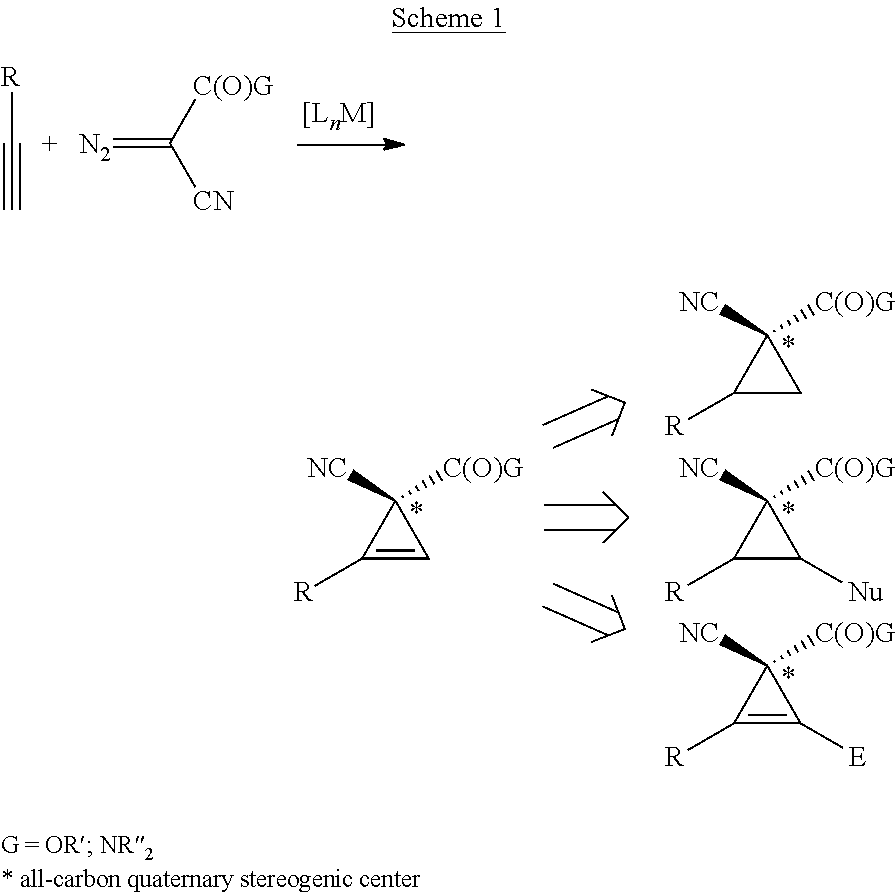
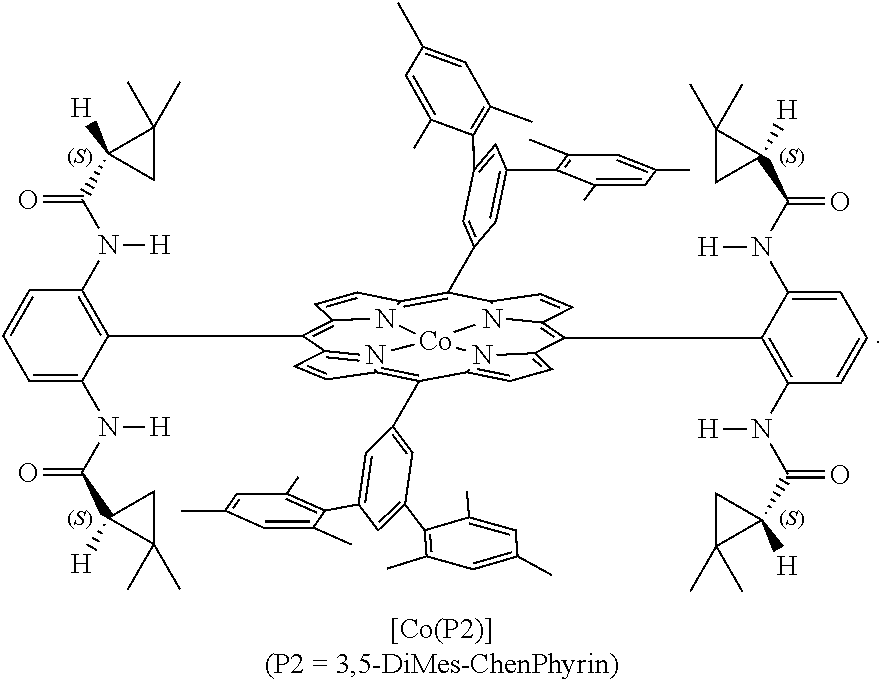
Enantioselective cyclopropenation of alkynes
InactiveUS20130030170A1Improve efficiencyHigh enantioselectivityHydrocarbon by isomerisationCarboxylic acid nitrile preparationPorphyrinAlkyne
The cobalt(II) complex of new D2-symmetric chiral porphyrin 3,5-DiMes-ChenPhyrin, [Co(P2)], has been shown to be a highly effective chiral metalloradical catalyst for enantioselective cyclopropenation of alkynes with acceptor / acceptor-substituted diazo reagents such as α-cyanodiazoacetamides and α-cyanodiazoacetates. The [Co(P2)]-mediated metalloradical cyclopropenation is suitable to a wide range of terminal aromatic and related conjugated alkynes with varied steric and electronic properties, providing the corresponding tri-substituted cyclopropenes in high yields with excellent enantiocontrol of the all-carbon quaternary stereogenic centers. In addition to mild reaction conditions, the Co(II)-based metalloradical catalysis for cyclopropenation features a high degree of functional group tolerance.
Owner:UNIV OF SOUTH FLORIDA
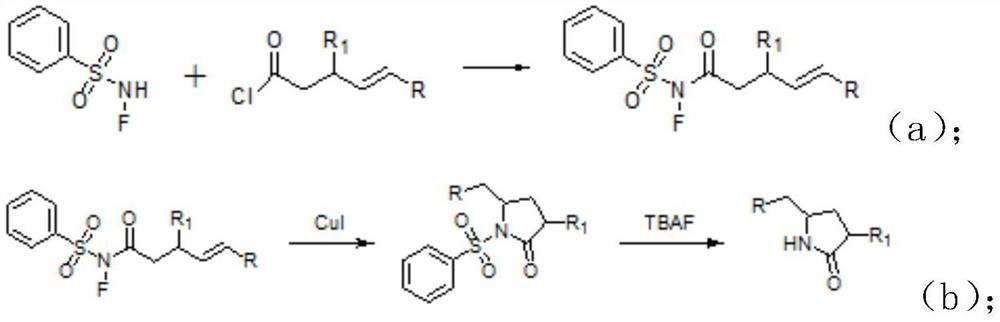
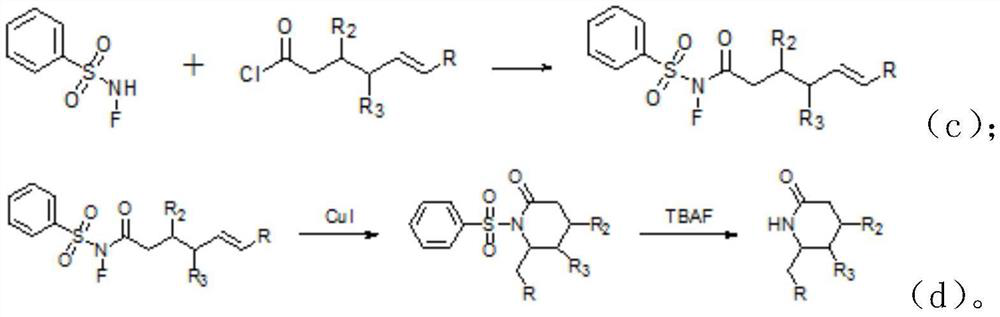
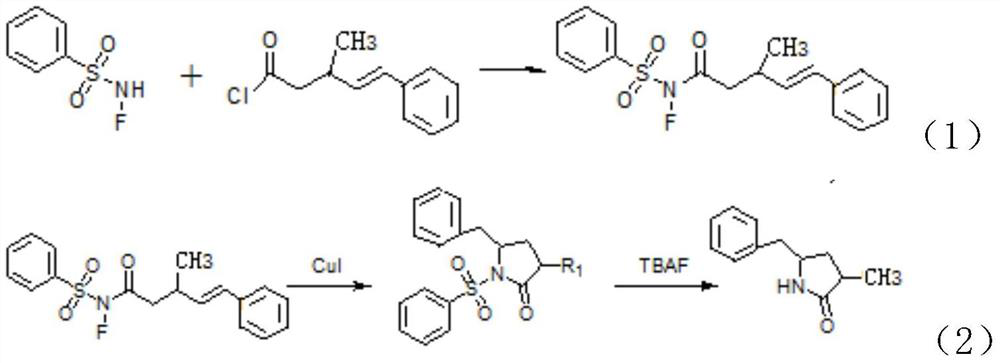
Synthetic method of lactam compound
PendingCN113045469AMild reaction conditionsReduce usageOrganic chemistryPtru catalystPhenylsulfonamide
The invention discloses a synthesis method of a lactam compound. The synthesis method comprises the following steps: (1) dissolving N-F benzenesulfonamide and acyl chloride in an organic solvent under the protection of gas, conducting uniform stirring, and conducting reacting to obtain a crude product; (2) recrystallizing the crude product to obtain a product A; (3) dissolving the product A obtained in the step (2), cuprous iodide and phenanthroline in a mixed organic solvent, and conducting heating for a reaction to obtain an intermediate; and (4) dissolving the intermediate, adding tetrabutylammonium fluoride, and performing stirring to obtain a target product. According to the invention, commercially available N-F benzenesulfonamide and acyl chloride are used as starting raw materials, and the materials are easy to obtain; according to the synthesis method disclosed by the invention, the commercially cheap and easily available cuprous iodide is selected as a catalyst, so the use of an expensive noble metal catalyst is avoided, and economical efficiency is effectively improved; the whole synthesis method disclosed by the invention is mild in reaction conditions; and the target compound is obtained through free radical cyclization, the use of other oxidizing agents or noble metal catalysts is avoided, and synthesis cost is low.
Owner:SHANGHAI WOKAI BIOTECH
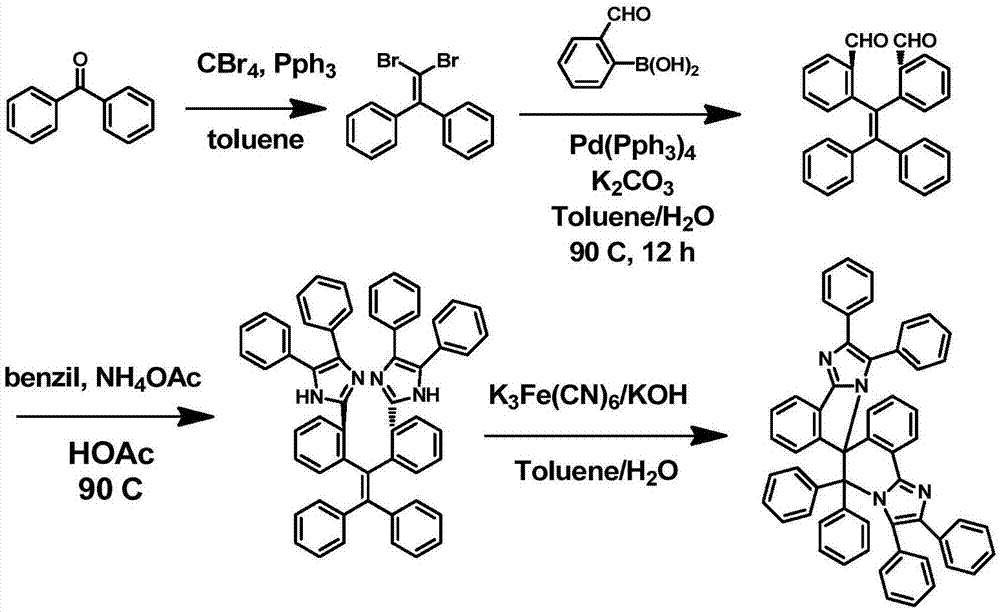
Spiro biimidazole molecule as well as preparation method and application thereof
ActiveCN106995444AFast fadingSolving the technical problem of biimidazole molecules lacking phosphorescent light emissionOrganic chemistryTenebresent compositionsBenzilCorey–Fuchs reaction
The invention discloses a preparation method and application of a spiro biimidazole molecule and a relevant derivative. Diphenyl ketone or a derivative thereof is taken as a raw material, and a corresponding dibromo aromatic ring olefin product is prepared by Corey-Fuchs reaction; then the product and o-formaldehyde phenylboronic acid generate Suzuki reaction to obtain geminal-ortho aldehyde substituted tetraryl ethylene; then the geminal-ortho aldehyde substituted tetraryl ethylene generates Leuckart reaction with benzil or a derivative thereof in acetic acid and ammonium acetate to obtain geminal-ortho tetraphenyl ethylene-triphenyl imidazole; finally, under the oxidation of K3Fe(CN)6 / KOH, the spiro biimidazole molecule is obtained by free radical cyclization addition. The molecule shows phosphorescence emitting behavior with longer service life (1.6 seconds) under low temperature (77K). The 3D spiro molecule may provide guidance for preparing a long-service-life room temperature phosphorescence small organic molecule material.
Owner:HUAZHONG UNIV OF SCI & TECH
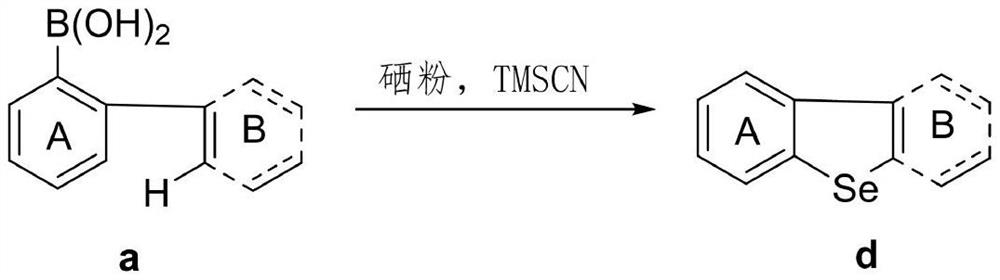
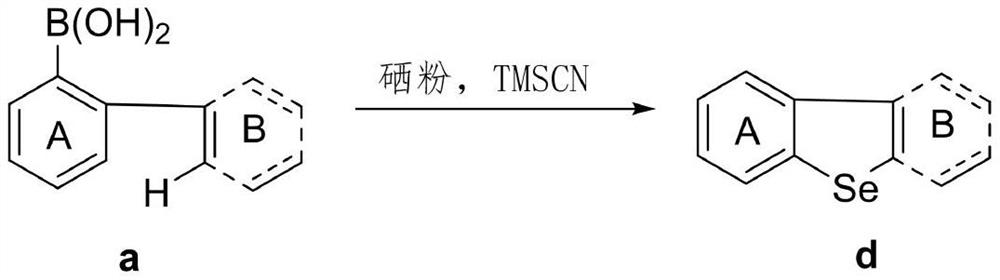
Synthetic method of dibenzoselenophene compound
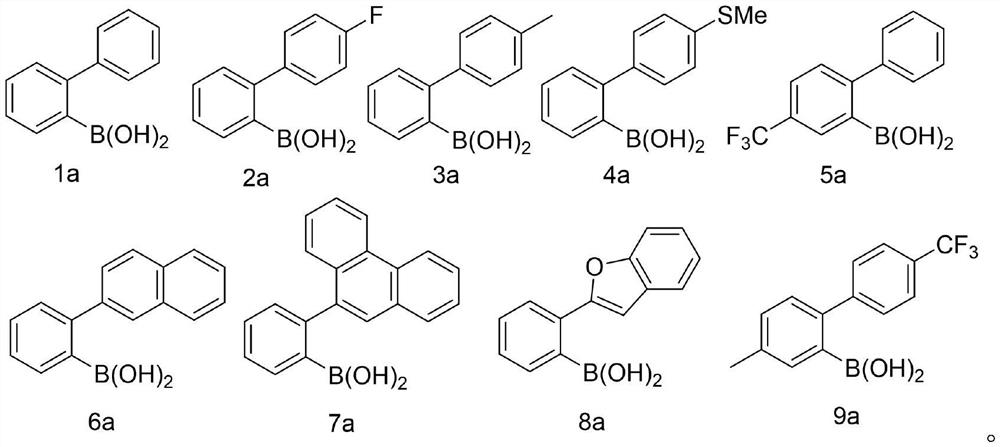
The invention discloses a synthetic method for preparing a dibenzoselenophene compound. According to the method, a C-Se bond is formed through free radical cyclization of a biaryl boric acid substratecatalyzed by TMSCN and selenium powder, so that the dibenzoselenophene compound is constructed. The new strategy has the advantages of no metal participation, no additive promotion, wide substrate range, good functional group compatibility, simple operation, high yield and the like.
Owner:WENZHOU UNIVERSITY
Preparation method of alkane C (sp3)-H functionalization started polycyclic quinazolinone derivative in water phase
The invention relates to a preparation method of a polycyclic quinazolinone derivative started by alkane C (sp3)-H functionalization in a water phase, in particular to a novel method for effectively synthesizing polycyclic quinazolinone by carrying out C (sp3)-H functionalization on alkane to generate alkyl free radicals and then cyclizing the free radicals of olefin substituted quinazolinone. Adding the raw materials, namely a quinazolinone compound, alkane, a phase transfer agent and an oxidizing agent into a solvent in an air atmosphere, and stirring at a certain reaction temperature to react, so as to generate the polycyclic quinazolinone derivative. The method has the advantages of wide application range of reaction substrates, greenness and high efficiency, and is particularly suitable for industrial production.
Owner:HUNAN POLICE ACAD
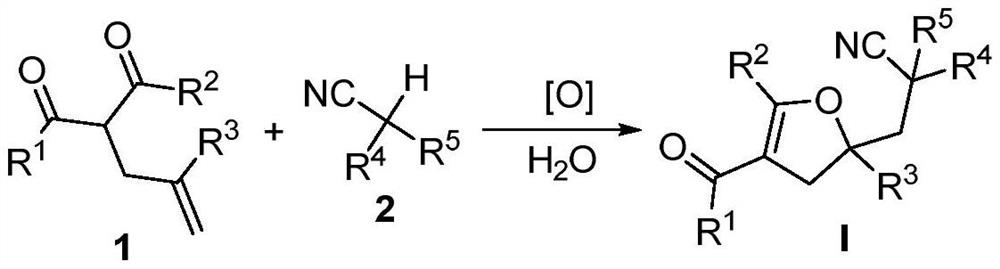
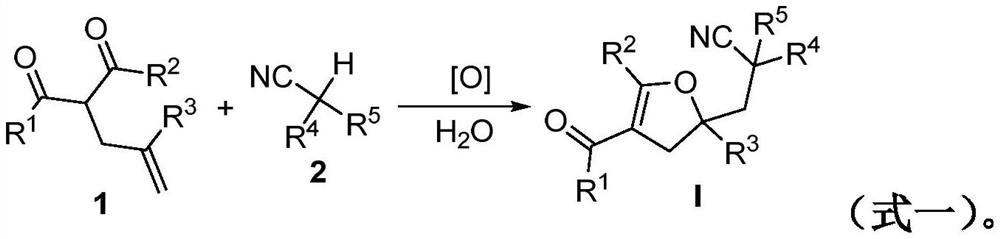
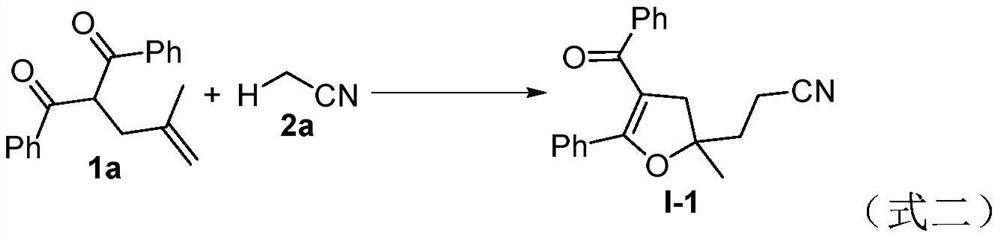
Method for preparing a 2, 3-dihydrofuran derivative
The invention discloses a method for preparing a 2, 3-dihydrofuran derivative through a nitrile C (sp3)-H functionalization initiated alkenyl-1, 3-dicarbonyl compound free radical cyclization reaction under a metal-free and alkali-free water phase system. The method comprises the following steps: adding an alkenyl-1, 3-dicarbonyl compound, a nitrile compound, an oxidizing agent and water into a Schlenk reaction flask, and stirring and reacting at a certain temperature in an air atmosphere to obtain the 2, 3-dihydrofuran derivative.
Owner:HUAIHUA UNIV
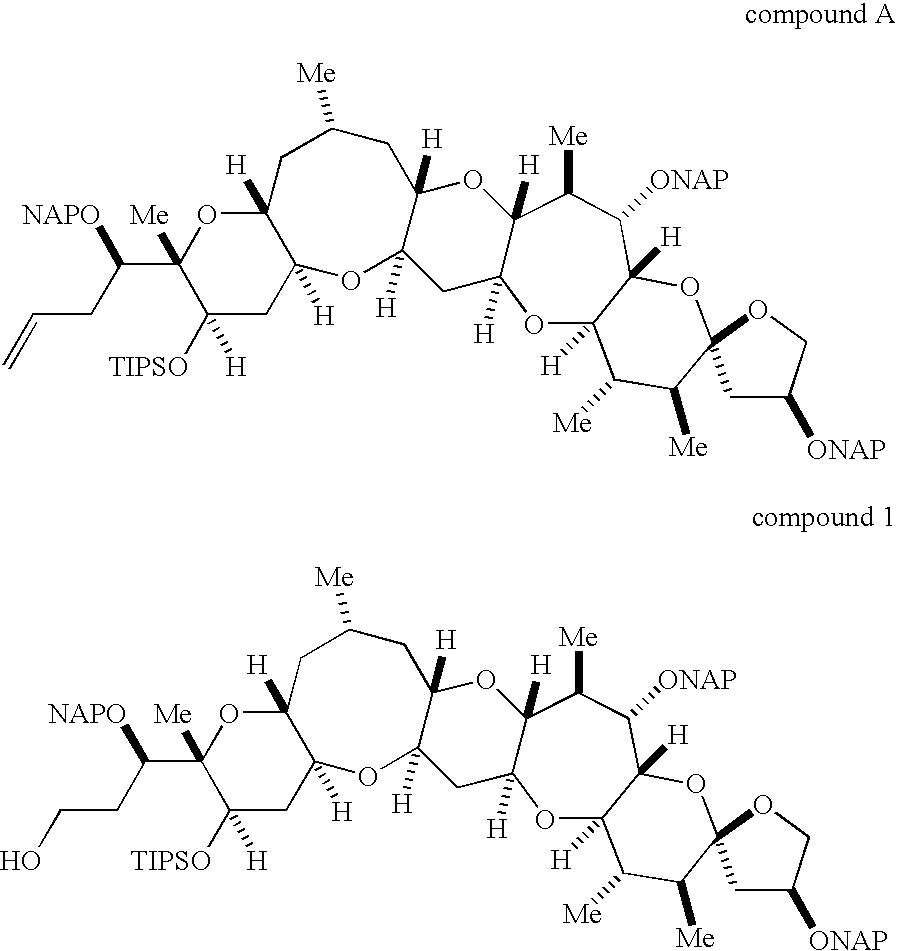
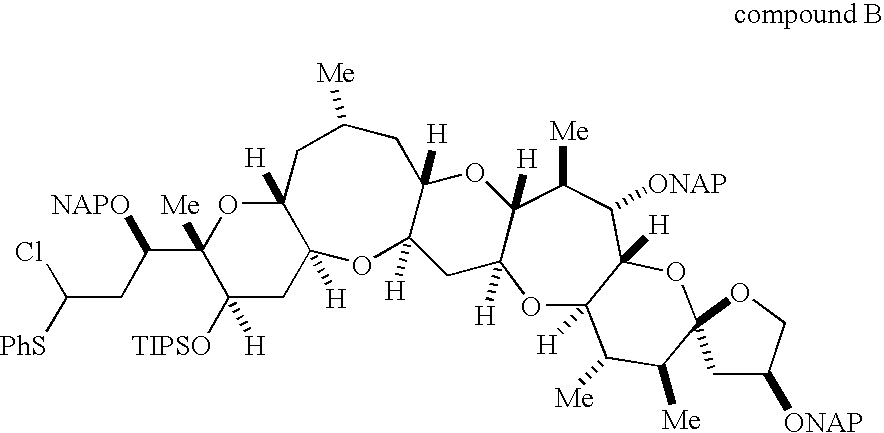
Method for synthesis of ciguatoxin CTX1B and compounds useful for the synthesis of ciguatoxin CTX1B
Disclosed is a method for total synthesis of CTX1B, which is developed for the synthesis of a ciguatoxin analogue such as CTX3C and enables the more efficient application of an established reaction to the total synthesis of CTC1B. More specifically, disclosed is a method for total synthesis of CTX1B comprising; an O.S-acetal formation for synthesizing a novel compound (3); a radical cyclization reaction for constructing a 9-membered ring formation reaction including a novel compound (6) through a novel compound (8) and yielding a compound (D); and a deprotection for yielding CTX1B. Also disclosed are novel compounds (1) to (8) which are particularly useful for synthesis of CTX1B and can be used for the synthesis of a ciguatoxin analogue.
Owner:JAPAN SCI & TECH CORP
Free radical cyclization reaction method based on 1,6-enynes and alcohols
InactiveCN110054578BSuitable for industrial productionNo heavy metal residueOrganic chemistryAlcohol freeAir atmosphere
The invention discloses a free radical cyclization reaction method based on 1,6-enynes and alcohol compounds, which belongs to the technical field of organic synthesis. The method comprises the following steps: using alcohol compounds as starting materials, Carry out cyclization reaction with 1,6-enyne compound, after the reaction is complete, post-treatment to obtain cyclization product 2-pyrrolidone compound. The method has cheap and easy-to-obtain raw materials, wide sources, stability and low toxicity, does not use any transition metal catalysts and ligands, and the reaction can be carried out in an air atmosphere with mild conditions, easy operation, no heavy metal residue in the product, and a wide range of reaction substrates. , Simple, efficient, economical and green, suitable for industrial production. At the same time, the present invention also provides an economical, practical and environmentally friendly new idea for the cyclization of alcohol radicals, expanding its application range.
Owner:YANGTZE NORMAL UNIVERSITY
1,3-disubstituted indoline derivative and preparation method thereof
The invention relates to a 1,3-disubstituted indoline derivative and a preparation method thereof, and belongs to the field of organic compound synthesis. The preparation method comprises following steps: step one, according to a mole ratio of (1-3): (2-10): 1: (3-8), in the absence of oxygen, dissolving SmI2, tertiary amine, allyl substituted 2-iodo aromatic amine, and deionized water by tetrahydrofuran to obtain a mixed system A; step two, making the mixed system carry out reactions for 0.5 to 20 minutes at a temperature of 20 to 30 DEG C, and removing the organic solvent and byproducts in the obtained reaction liquid to obtain the 1,3-disubstituted indoline derivative. Water is used as a proton donor, the reactions are triggered by samarium iodide, and under the catalytic action, cyclization of free radicals happens in tetrahydrofuran. The target product is obtained under mild conditions. The reaction speed is quick, the conditions are mild, and the obtained 1,3-disubstituted indoline derivative can be widely used in fields such as medicine, pesticide, and the like.
Owner:SHAANXI UNIV OF SCI & TECH
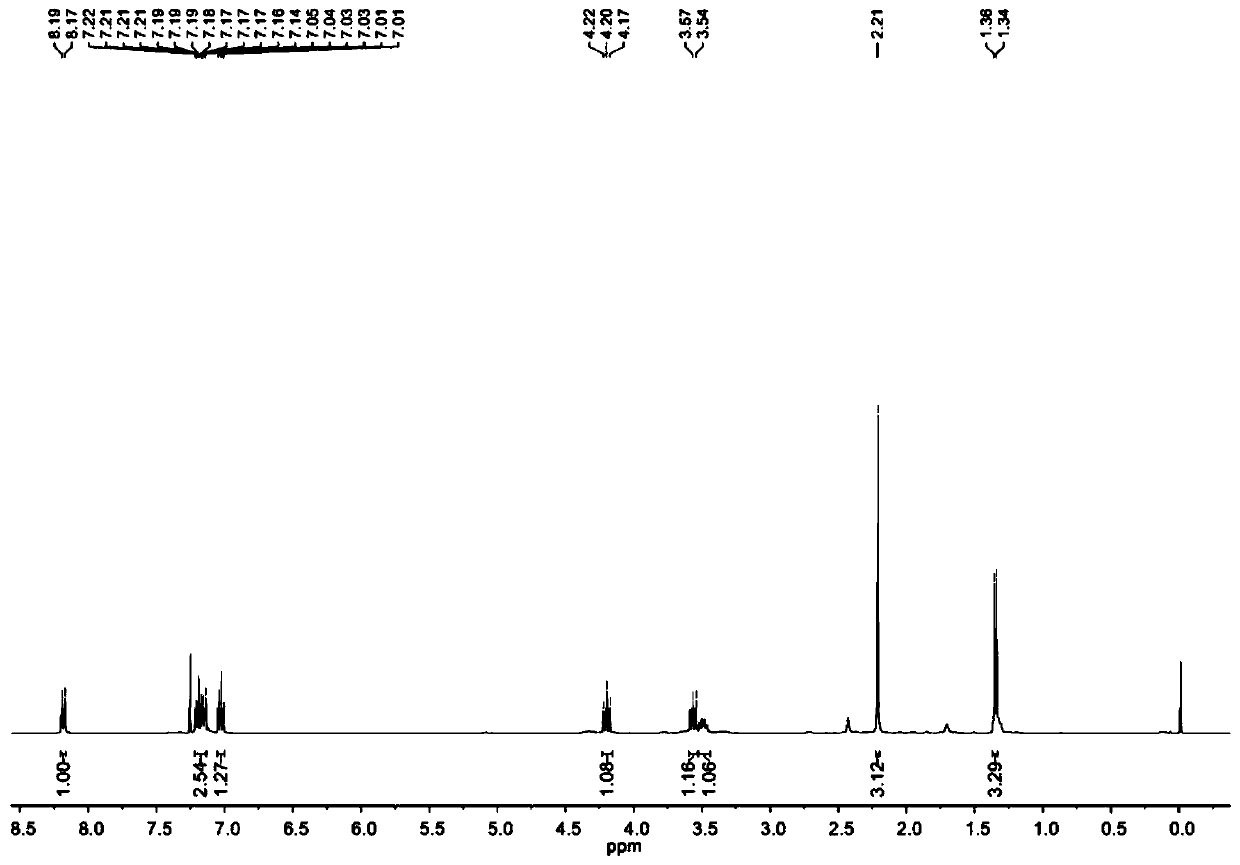
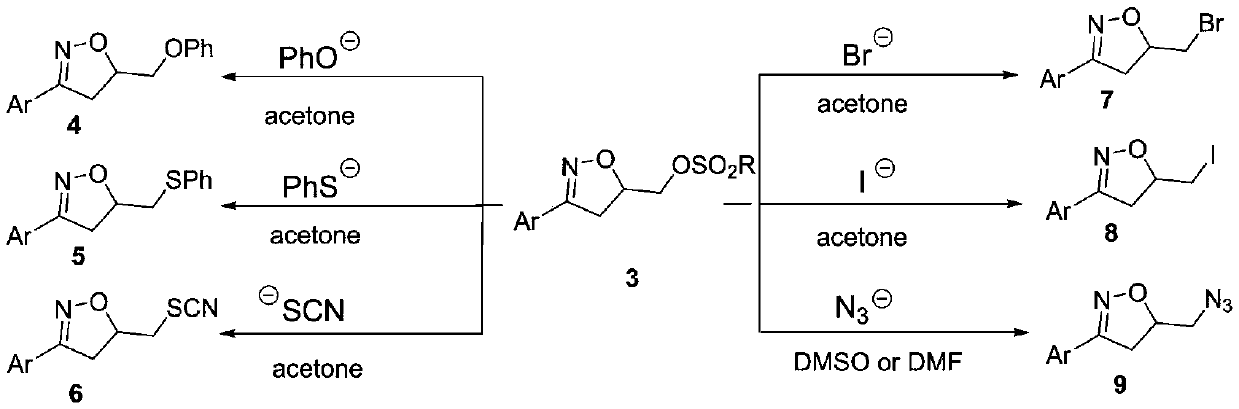
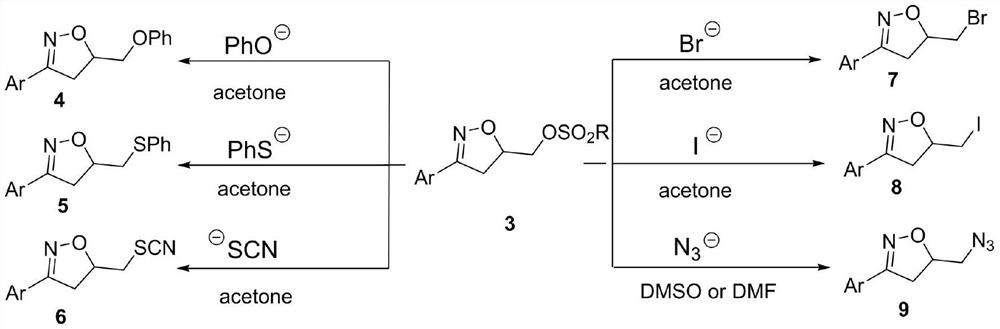
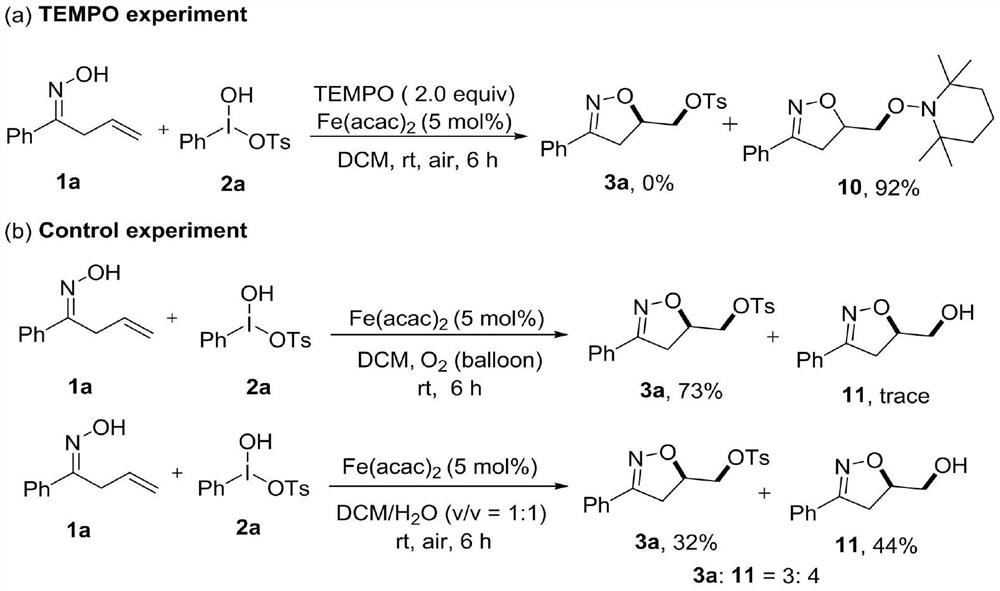
Synthesis method of 3-aryl-4,5-dihydroisoxazole-5-yl-methyl sulfonate and analogs
The invention discloses a synthesis method of 3-aryl-4,5-dihydroisoxazole-5-ylmethyl p-toluenesulfonate and analogs, belonging to the technical field of organic chemistry. Iron (II) is used to catalyze Koser high iodine reagent (HIRS) to promote I-O bond cleavage and unsaturated oxime radical cyclization, thus obtaining 3-phenyl-4,5-dihydroisoxazole-5-yl sulfonate, an isoxazoline skeleton product.Sulfonate groups as leaving groups can be further converted into various series of analogs. The method provides a simple, cheap and efficient way to synthesize the 3-aryl-4,5-dihydroisoxazole-5-ylmethyl sulfonate series.
Owner:HUAIBEI NORMAL UNIVERSITY
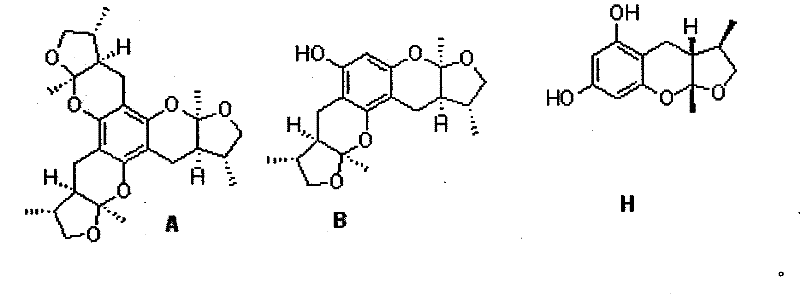
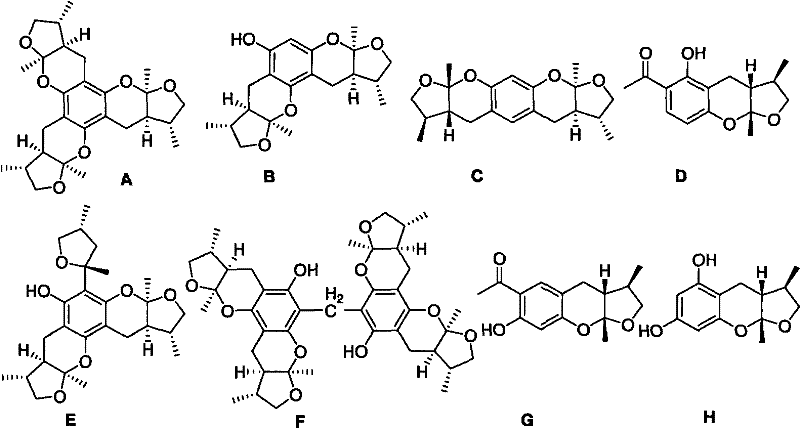
Synthetic method of Xyloketals compounds
Owner:SUN YAT SEN UNIV
Synthetic method of visible light promoted polycyclic quinazolinone compound initiated by N-hydroxyphthalimide ester decarboxylation
The invention relates to a synthetic method of a polycyclic quinazolinone compound initiated by N-hydroxyphthalimide ester decarboxylation promoted by visible light. The method comprises the following steps: in a nitrogen atmosphere, putting a quinazolinone compound, an N-hydroxyphthalimide ester compound, an iodine additive and a ligand into a solvent, and synthesizing the polycyclic quinazolinone product through oxidative decarboxylation and free radical cyclization reaction under the driving of visible light. The method is high in yield, has the advantages of being wide in substrate application range, mild in reaction condition, economical, environment-friendly, green and efficient, and is particularly suitable for industrial production.
Owner:HUNAN POLICE ACAD
A kind of synthetic method of 3-aryl-4,5-dihydroisoxazol-5-yl methylsulfonate and analogs
The invention discloses a method for synthesizing 3-aryl-4,5-dihydroisoxazol-5-ylmethyl p-toluenesulfonate and analogues, belonging to the technical field of organic chemistry. Using iron(II) to catalyze Koser hypervalent iodine reagents (HIRs) to promote the cleavage of I-O bonds and cyclization of unsaturated oxime radicals to obtain the isoxazoline skeleton product 3-phenyl-4,5-dihydroisoxane Azol-5-yl sulfonate. The sulfonate group acts as a leaving group, which can be further transformed into a variety of series of analogs. This method provides a simple, cheap and efficient way for the synthesis of 3-aryl-4,5-dihydroisoxazol-5-yl methanesulfonate series.
Owner:HUAIBEI NORMAL UNIVERSITY
Free radical cyclization reaction method of 1, 6-diene and azoalkyl nitrile in water phase
The invention discloses a free radical cyclization reaction method of 1, 6-diene and azoalkyl nitrile in a water phase, and relates to a high-regioselectivity free radical cyclization reaction methodof a 1, 6-diene compound and azoalkyl nitrile in a water phase without any additive. The method comprises the following steps of: adding a 1, 6-diene compound, an azo alkyl nitrile compound and waterinto a Schlenk reaction flask, and carrying out a stirring reaction at a certain temperature in an air atmosphere to obtain a cyclized product.
Owner:NINGBO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
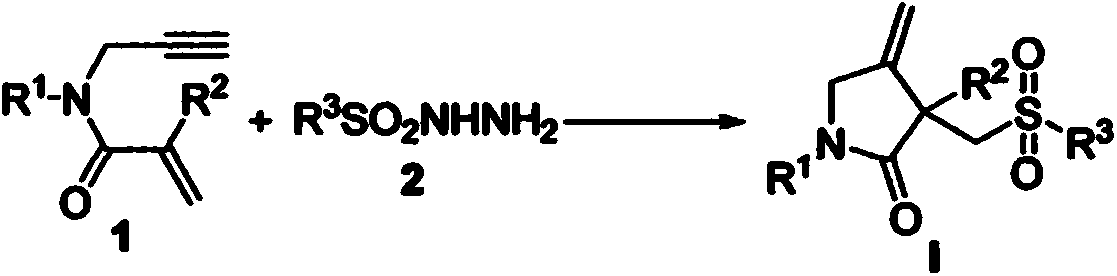
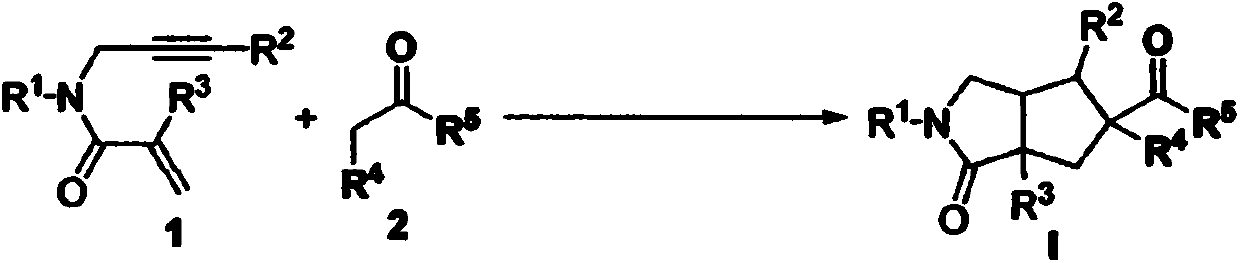
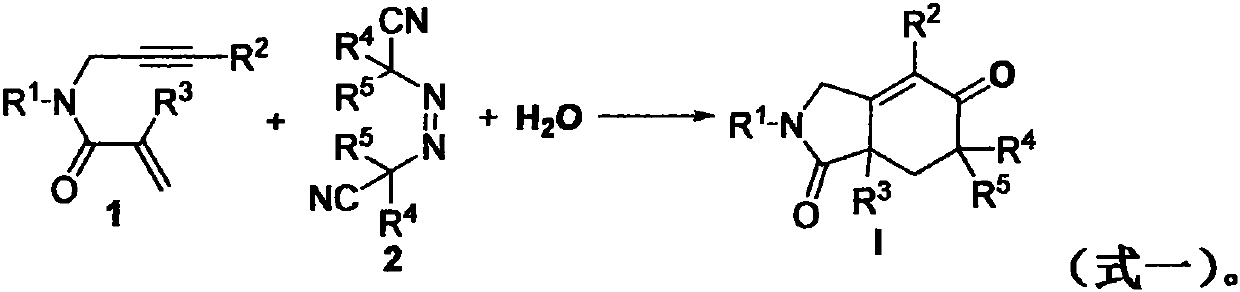
![Benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone and derivative and preparation method thereof Benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone and derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/8456ee6c-934c-497d-8c97-68b52e8f31d9/HDA0003213040030000011.png)
![Benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone and derivative and preparation method thereof Benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone and derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/8456ee6c-934c-497d-8c97-68b52e8f31d9/FDA0003213040010000011.png)
![Benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone and derivative and preparation method thereof Benzo [4, 5] imidazo [2, 1-a] isoquinoline-6 (5H)-ketone and derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/8456ee6c-934c-497d-8c97-68b52e8f31d9/FDA0003213040010000013.png)