Synthetic method of dibenzoselenophene compound
A technology of dibenzoselenophene and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of low yield of target product, harsh reaction conditions, complexity, etc., and achieve good functional group compatibility, wide substrate range, and no metal participation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0021] Embodiment 1-10 Reaction condition optimization embodiment
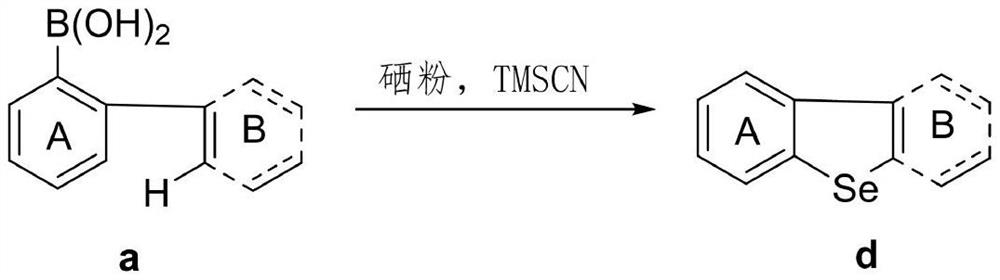
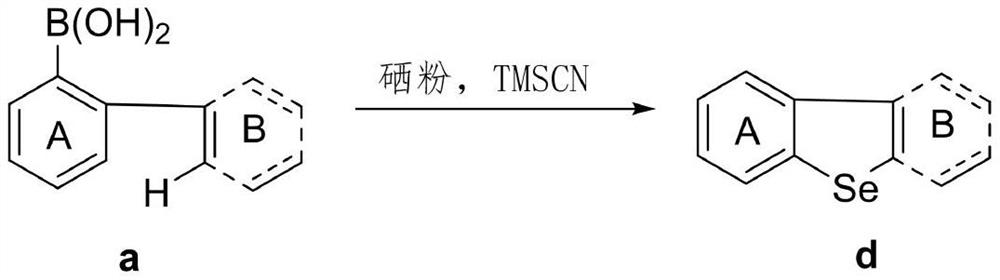
[0022] Using [1,1'-biphenyl]-2-ylboronic acid represented by formula 1a as the template substrate, the optimal reaction conditions were screened. The reaction formula is as follows:
[0023]
Embodiment 1
[0025] [1,1'-biphenyl]-2-ylboronic acid (0.2mmol), Se (0.6mmol) and DMSO (2ml) shown in Formula 1a were loaded into a 25mL Schlenk tube equipped with a stirring bar, and the reaction mixture Stir at 140° C. for 24 h under an air atmosphere, take a sample and detect no reaction by TLC and / or GC-MS.
Embodiment 2
[0027] [1,1'-biphenyl]-2-ylboronic acid (0.2mmol), TMSCN (1mol%, ie 0.002mmol), Se (3 eq. , ie 0.6mmol) and DMSO (2ml). The reaction mixture was stirred at 140° C. for 24 h under an air atmosphere. After cooling, the reaction mixture was diluted with 10 mL of ether, filtered through a pad of silica gel and concentrated under reduced pressure. Then the residue was purified by silica gel flash chromatography to obtain the target product phenoxeselene / phenothioselenium represented by formula 1d with a yield of 40%. white solid; 1 H NMR (500MHz, CDCl 3 )δ8.24-8.23(m,2H),8.06-8.04(m,2H),7.62-7.59(m,2H),7.56-7.53(m,2H); 13 C NMR (125MHz, CDCl 3 )δ 139.6, 138.5, 127.1, 126.3, 125.1, 123.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com