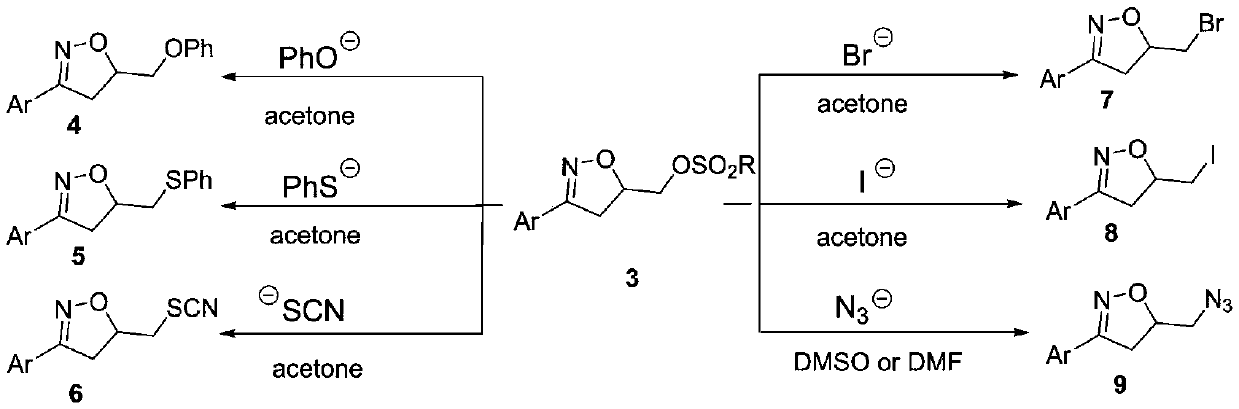
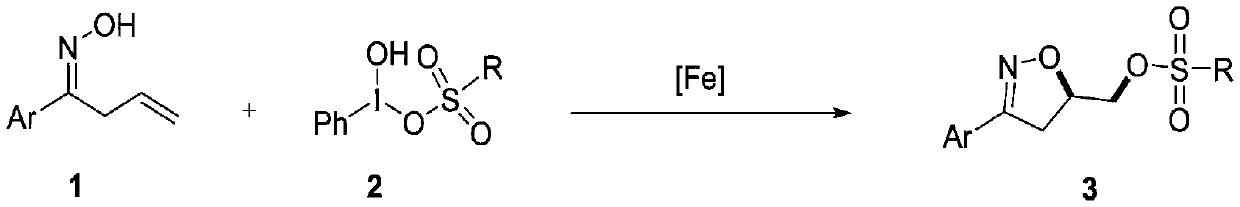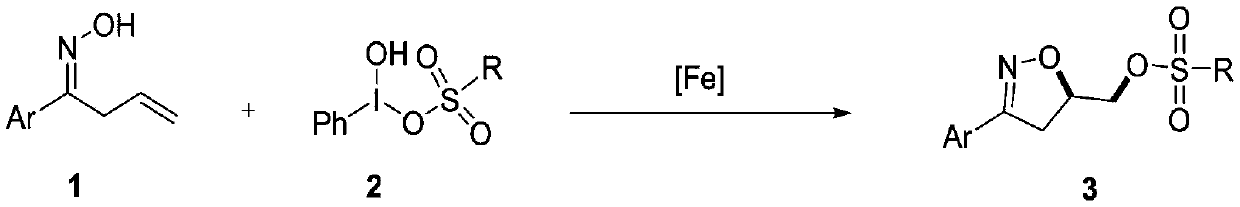Synthesis method of 3-aryl-4,5-dihydroisoxazole-5-yl-methyl sulfonate and analogs
A kind of technology of methyl methanesulfonate and dihydroisoxazole, applied in the synthesis field of isoxazole heterocycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Optimization of reaction conditions
[0029] (E)-1-phenylbut-3-ene-1-oxime (1a, 32.2mg, 0.2mmol), iron catalyst (5mol%), organic solvent (1.0mL) and [hydroxyl (p-toluenesulfonyloxy ) iodo]benzene (2a, 98.1mg, 0.25mmol) was sequentially added into a 10mL reaction tube. The reaction tube was stirred at room temperature, and then the mixture in the reaction tube was detected by TLC. After the reaction was completed, distilled water (10 mL) was added and extracted with an organic solvent. The organic layers were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product, which was purified by flash chromatography (silica gel, petroleum ether / ethyl acetate=3:1-9:1) to obtain product 3a.
[0030]
[0031]
[0032] In the screening process of reaction conditions, no catalyst was added (label 1), and the influence of different iron catalysts on the reaction (label 2-8), the influence of different reaction solvents on...
Embodiment 2
[0034] (E)-1-phenylbut-3-ene-1-oxime (1a, 32.2mg, 0.2mmol), Fe(acac) 2 (2.5 mg, 5 mol%), DCM (1.0 mL) and [hydroxy(p-toluenesulfonyloxy)iodo]benzene (2a, 98.1 mg, 0.25 mmol) were sequentially added to a 10 mL reaction tube. The reaction tube was stirred at room temperature for 6 h, and then the mixture in the reaction tube was detected by TLC. After the reaction was completed, distilled water (10 mL) was added, and extracted with dichloromethane (DCM, 3×10 mL). The organic layers were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product, which was purified by flash chromatography (silica gel, petroleum ether / ethyl acetate=3:1~9:1) to obtain 53.7 mg of product 3a, white Solid, yield 81%.
[0035]
[0036] (3-Phenyl-4,5-dihydroisoxazol-5-yl)methyl 4-methylbenzenesulfonate(3a):White solid; 53.7mg, 81% yield; 1 H NMR (600MHz, CDCl 3)δ7.79(d, J=8.4Hz, 2H), 7.63–7.61(m, 2H), 7.44–7.38(m, 3H), 7.34(d, J=7.8Hz, 2H), 4....
Embodiment 3
[0038] According to the reaction conditions of Example 2, only the structure of the substrate 1 was changed to obtain the reaction product 3b-3s, and the specific results are as follows:
[0039]
[0040] (3-(p-Tolyl)-4,5-dihydroisoxazol-5-yl)methyl
[0041] 4-methylbenzenesulfonate (3b): White solid; 53.9mg, 78% yield; 1 H NMR (400MHz, CDCl 3 )δ7.79(d, J=8.4Hz, 2H), 7.50(d, J=8.0Hz, 2H), 7.34(d, J=8.0Hz, 2H), 7.20(d, J=8.0Hz, 2H) ,4.94–4.87(m,1H),4.16–4.08(m,2H),3.42(dd,J=17.2,10.8Hz,1H),3.21(dd,J=16.8,6.8Hz,1H),2.44(s ,3H),2.38(s,3H). 13 C NMR (101MHz, CDCl 3 )δ156.2, 145.2, 140.7, 132.5, 129.9, 129.4, 128.0, 126.7, 126.0, 77.2, 69.2, 37.4, 21.6, 21.4. HRMS (ESI) ([M+H] + )Calcd.For[C 18 h 20 NO 4 S] + :346.1108,Found:346.1108.
[0042]
[0043] (3-(4-Ethylphenyl)-4,5-dihydroisoxazol-5-yl)methyl
[0044] 4-methylbenzenesulfonate (3c): White solid; 53.9mg, 75% yield; 1 H NMR (400MHz, CDCl 3 )δ7.79(d, J=8.4Hz, 2H), 7.53(d, J=8.4Hz, 2H), 7.34(d, J=8.0Hz, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com