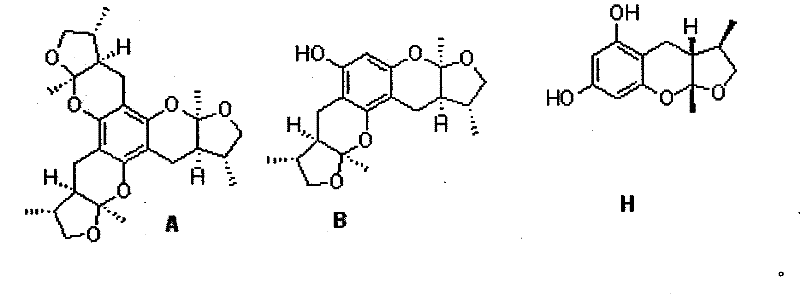
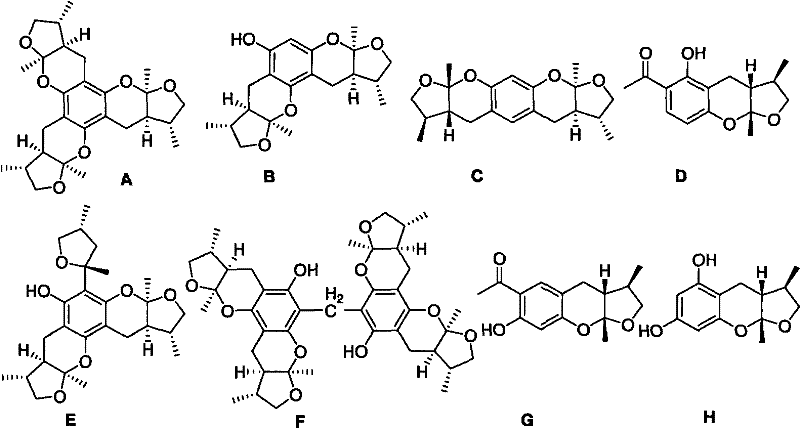
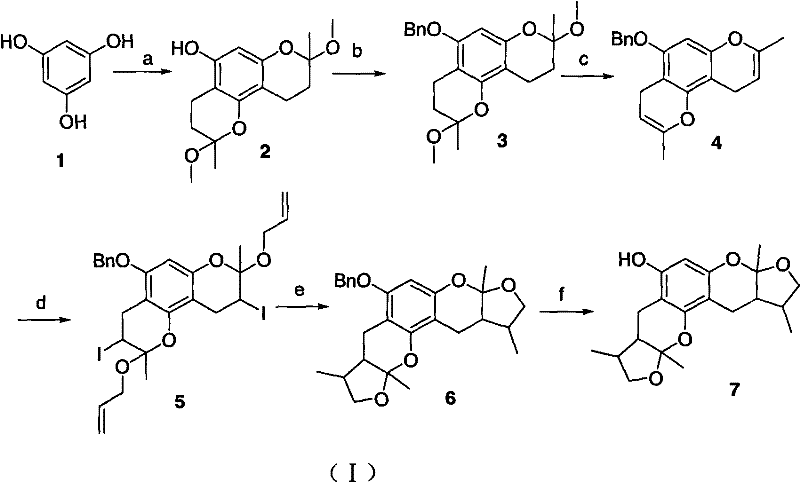
Synthetic method of Xyloketals compounds
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve the problems of complex process and high production cost, and achieve the effect of simple process, low production cost and product purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of three substituted benzopyran ring ethers
[0023] In a 50ml round bottom flask, add 1.0g (7.94mmol) phloroglucinol and 2.2g (31.75mmol) butenone, 2.0g anhydrous magnesium sulfate, then add 30ml of anhydrous methanol, stir at 0°C to dissolve it, Then slowly add 2.56g (15.88mmol) of p-toluenesulfonic acid, continue to stir the reaction, let it slowly rise to room temperature, react for 8h, TLC after the reaction is completed, add saturated NH4Cl 10ml at 0°C to stop the reaction, add 100ml EtOAc, saturated NH 4 Cl (1×10ml), washed with saturated brine (2×10ml), anhydrous MgSO 4 It was dried and concentrated under reduced pressure. Column chromatography gave 2.85 g of white solid with a yield of 95%. mp 134-135°C; 1 H NMR (CDCl 3 , 400MHz) δ: 3.255(s, 3H), 3.242(s, 3H), 3.237(s, 3H), 2.641(m, 6H), 2.058(m, 3H), 1.746(m, 3H), 1.524(s , 6H), 1.518(s, 3H);
Embodiment 2
[0025] 1. Synthesis of disubstituted benzopyran cyclic ether (2)
[0026] In a 50ml round bottom flask, add 1.0g (7.94mmol) phloroglucinol and 1.3g (18.26mmol) butenone, 1.5g anhydrous magnesium sulfate, then add 30ml anhydrous methanol, stir at 0°C to dissolve it, Then slowly add 1.43g (7.94mmol) of p-toluenesulfonic acid, continue to stir the reaction, let it slowly rise to room temperature, react for 8h, TLC after the completion of the tracking reaction, add saturated NH at 0°C 4 Cl 10ml, stop the reaction, add 100ml EtOAc, saturated NH 4 Cl (1×10ml), washed with saturated brine (2×10ml), anhydrous MgSO 4 It was dried and concentrated under reduced pressure. Column chromatography gave 1.68 g of white solid with a yield of 72%. mp 170-171°C, 1 H NMR (CDCl 3 , 400MHz) δ: 5.977(s, 1H), 5.167(s, 1H), 3.270(s, 3H), 3.260(s, 3H), 2.649(m, 2H), 2.604(m, 2H), 2.101(m , 1H), 2.069(m, 1H), 1.792(m, 1H), 1.730(m, 1H), 1.542(s, 3H), 1.518(s, 3H);
[0027] 2. Protection of disubst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com