Preparation method of alkane C (sp3)-H functionalization started polycyclic quinazolinone derivative in water phase
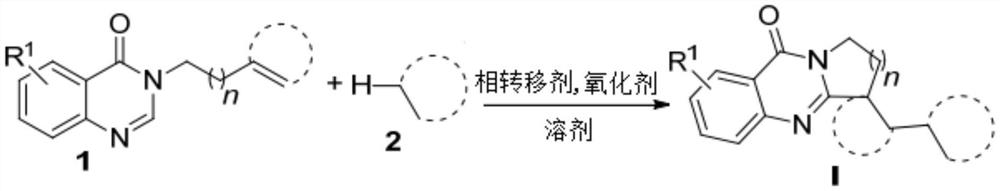
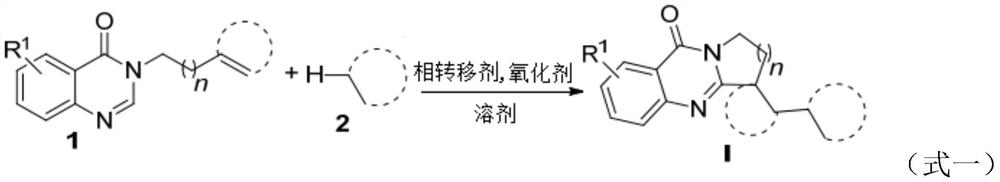
The technology of polycyclic quinazolinone and quinazolinone is applied in the field of preparation of polycyclic quinazolinone derivatives initiated by C-H functionalization of alkanes in aqueous phase, and can solve the problems of lengthy reaction procedures, complicated preparation of raw materials, Complex reaction conditions and other problems, to achieve a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
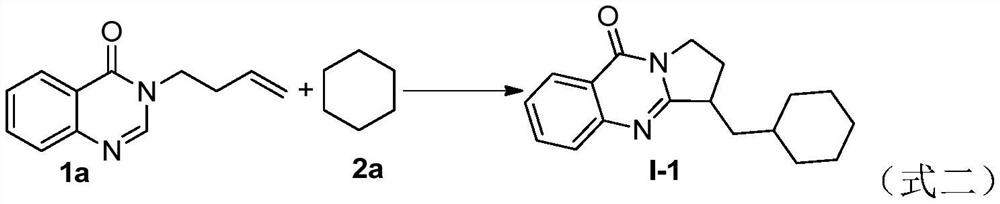
[0016] 40.0 mg (0.2 mmol) of the quinazolinone compound represented by formula 1a, 0.2 mL of cyclohexane represented by formula 2a, 58.5 mg (2.0 equiv) of di-tert-butyl peroxide, and 58.5 mg (2.0 equiv) of di-tert-butyl peroxide were added to the Schlenk reaction flask. 11.5 mg (0.2 equiv) of sodium alkyl sulfonate and 1.8 mL of water, then the reaction flask was stirred and reacted at 95° C. The reaction progress was monitored by TLC until the raw materials disappeared (the reaction time was 28 hours). After the reaction stopped, the reaction solution was extracted with ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to remove the solvent, and the residue was separated by column chromatography (elution solvent: ethyl acetate / normal Hexane=7:1) to obtain the target product (yield 87%), the product structural formula is shown in formula I-1, and the characterization data are: 1 H NMR (400MHz,...
Embodiment 2
[0018] The oxidant used tert-butyl peroxide (TBHP) instead of di-tert-butyl peroxide (DTBP), and other conditions were the same as in Example 1, and the yield of the target product I-1 was 71%.
Embodiment 3
[0020] The oxidizing agent used benzoyl peroxide (BPO) instead of di-tert-butyl hydroperoxide (DTBP), and other conditions were the same as those in Example 1, and the yield of the target product I-1 was 58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com