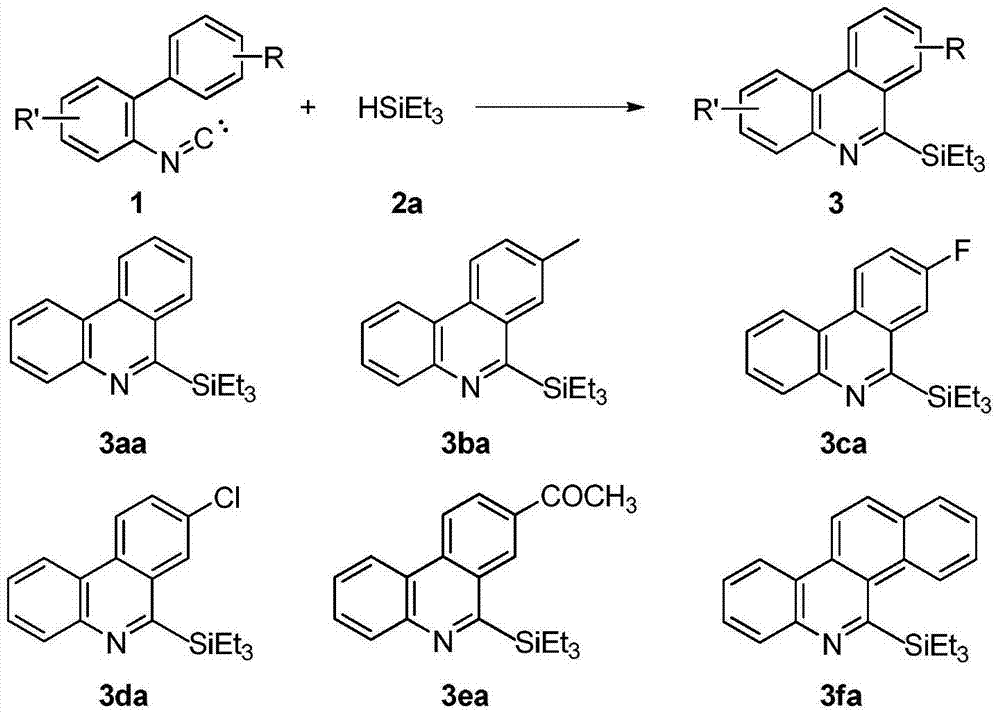
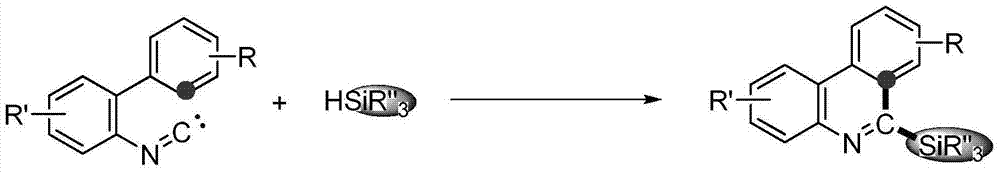Method for synthesizing phenanthridine silane derivative
A technology of phenanthridine silane and alkyl silane, applied in the field of synthesis of phenanthridine silane derivatives, can solve the problems of uneconomical reaction, expensive catalyst and the like, and achieve the effects of simple handling, efficient reaction and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Mix 2-phenylbenzeneisonitrile 1a (2mmol), triethylsilane 2a (2mmol) and free radical initiator tert-butyl hydroperoxide (2mmol), add 10mL of benzene as a solvent, and react at room temperature in air, the reaction time Go through 4 hours. The conversion of 2-phenylbenzeneisonitrile was 100%, and the yield of 6-(triethylsilyl)phenanthridine 3aa was 80%.
Embodiment 2
[0026] Mix 2-p-tolylbenzene isocyanide 1b (2mmol), triethylsilane 2a (10mmol) and free radical initiator tert-butyl hydroperoxide (2mmol), add toluene 10mL as solvent, and react at room temperature in air. The time passes 4 hours. The conversion of 2-p-tolylbenzonitrile was 100%, and the yield of 8-methyl 6-(triethylsilyl)phenanthridine 3ba was 80%.
Embodiment 3
[0028] Mix 2-p-fluorophenylbenzeneisonitrile 1c (2mmol), triethylsilane 2a (2mmol) and free radical initiator tert-butyl hydroperoxide (20mmol), add 10mL of fluorobenzene as solvent, and react at room temperature in air , the reaction time went through 12 hours. The conversion of 2-p-fluorophenylbenzeneisonitrile was 100%, and the yield of 8-fluoro6-(triethylsilyl)phenanthridine 3ca was 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com