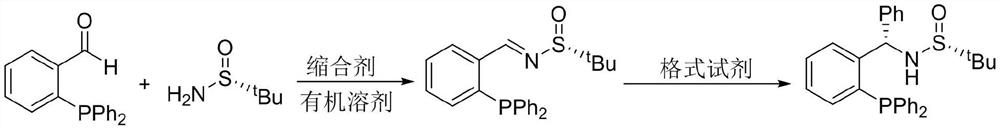
A large amount of preparation method of chiral sulfinamide monophosphine ligand
A technology for sulfenamide mono- and phosphine ligands, which is applied in the directions of organic chemistry methods, chemical instruments and methods, and compounds of Group 5/15 elements of the periodic table, etc. and other problems to achieve the effect of improving the synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
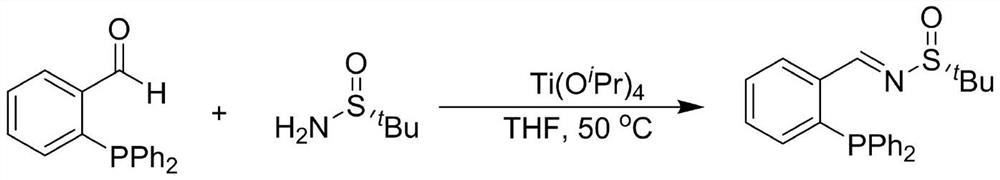
[0024] Example 1: Large-scale synthesis of chiral sulfinimides
[0025]
[0026] Wherein, THF is tetrahydrofuran; N 2 is nitrogen; Ti(O i Pr) 4 For tetraisopropyl titanate.
[0027] Accurately weigh o-diphenylphosphinebenzaldehyde (52.5mmol) and R-(+)-tert-butylsulfinamide (57.75mmol) in a 500mL three-necked reaction flask, and add 250mL of dry Tetrahydrofuran. Placed at 50° C. for 20 h. After the solvent temperature stabilized, tetraisopropyl titanate (157.5 mmol) was added dropwise through a constant-pressure low-liquid funnel as a condensation agent. After the complete reaction of the raw materials was detected by TLC, 100 mL of ethyl acetate was added to the reaction flask to dilute the solution. Then transfer the reaction solution to a 2L beaker filled with 200mL of saturated saline, and stir it rapidly with a glass rod for 5min to fully hydrolyze the tetraisopropyl titanate, and use a sand core funnel covered with diatomaceous earth to pump Filter, and wash the ...
Embodiment 2
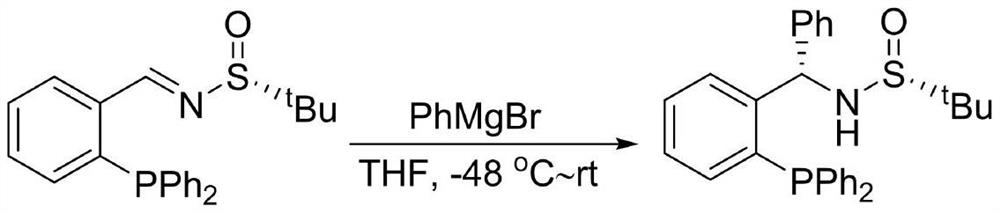
[0029] Embodiment 2: the synthesis of chiral sulfinamide monophosphine ligand
[0030]
[0031] Add imine (40mmol) into a dry 500mL three-necked reaction flask, add 250mL of redistilled tetrahydrofuran under nitrogen atmosphere, and place it at -48°C. After the solution temperature is completely stable, add 80mL of phenyl bromide The magnesium reagent was placed in a constant pressure funnel (80.0 mmol), and the rate of addition was controlled to 2 drops per second. After reacting for 2 hours, turn off the refrigeration and stir overnight, and then continue to stir at room temperature for 2 hours. After the reaction was detected by TLC, 100 mL of saturated ammonium chloride solution was added to quench the reaction. Transfer to a 1L separatory funnel, extract three times with 100mL ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and concentrate. The crude product was dissolved in 56 mL of acetone, then 40 mL of distilled water was added dropw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com