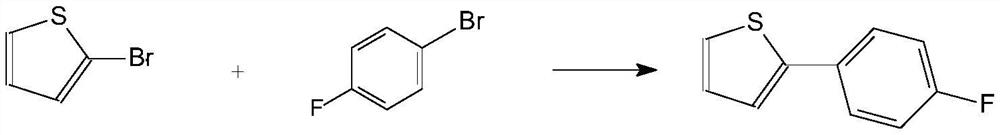
A kind of preparation method of high-purity 2-(4-fluorophenyl)thiophene
A technology of fluorophenyl and fluorophenylmagnesium bromide, which is applied in the field of preparation of high-purity 2-thiophene, can solve the problems of increased dosage, darker product color, increased cost, etc., and achieves reduced usage and improved reaction efficiency , the effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Under nitrogen conditions, magnesium chips (11.1g, 0.457mol), solvent tetrahydrofuran (140ml), a small amount of p-fluorobromobenzene (10g, 0.057mol) were added to a 500ml reaction flask, and the initiator iodine (0.1g) was added, stirred until The color faded. When the trigger was confirmed, the temperature was controlled at 36-40°C, and p-bromofluorobenzene (69.9g, 0.4mol) was added dropwise within 4h, controlled by GC. After the reaction was complete, the temperature was kept at 40°C, and the stirring was stopped.
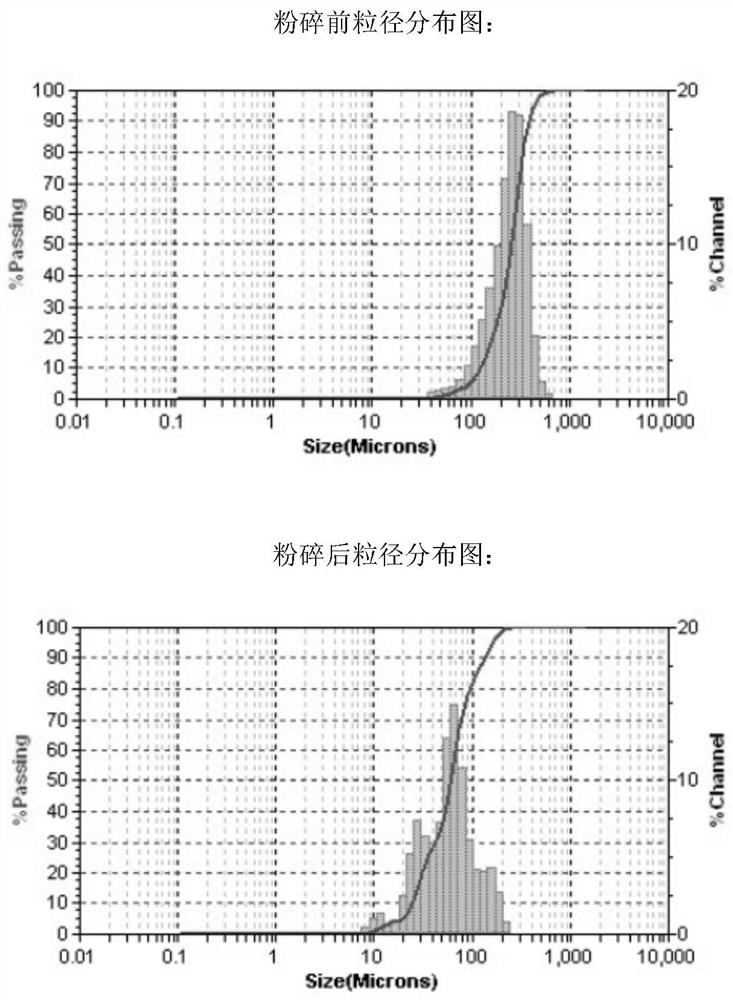
[0029] Take a 1000ml reaction bottle, add 2-bromothiophene (74.5g, 0.457mol) and solvent tetrahydrofuran (150ml) under the protection of nitrogen, replace with nitrogen three times, add catalyst DPPE.NICl2 (0.48g, 0.0009mol) (not sieved after grinding , particle size 0-250 μm), the temperature is controlled at 20-25 ° C, the Grignard reagent prepared above is added dropwise, and the drop is completed within 5 hours, controlled by GC. After the reaction wa...
Embodiment 2
[0031] Under nitrogen conditions, magnesium chips (11.1g, 0.457mol), solvent tetrahydrofuran (140ml), a small amount of p-fluorobromobenzene (10g, 0.057mol) were added to a 500ml reaction flask, and the initiator iodine (0.1g) was added, stirred until The color faded. When the trigger was confirmed, the temperature was controlled at 36-40°C, and p-bromofluorobenzene (69.9g, 0.4mol) was added dropwise within 4h, controlled by GC. After the reaction was complete, the temperature was kept at 40°C, and the stirring was stopped.
[0032] Take a 1000ml reaction bottle, add 2-bromothiophene (74.5g, 0.457mol) and solvent tetrahydrofuran (150ml) under the protection of nitrogen, replace nitrogen three times, add catalyst DPPE.NICl2 (0.48g, 0.0009mol) and 260-mesh sieve, particle size 57-125μm), temperature control 20-25°C, dropwise add the above-prepared Grignard reagent, 5h drop complete, GC central control. After the reaction was complete, it was quenched with 5% dilute hydrochloric ...
Embodiment 3
[0034] Under nitrogen conditions, magnesium chips (11.1g, 0.457mol), solvent tetrahydrofuran (140ml), a small amount of p-fluorobromobenzene (10g, 0.057mol) were added to a 500ml reaction flask, and the initiator iodine (0.1g) was added, stirred until The color faded. When the trigger was confirmed, the temperature was controlled at 36-40°C, and p-bromofluorobenzene (69.9g, 0.4mol) was added dropwise within 4h, controlled by GC. After the reaction was complete, the temperature was kept at 40°C, and the stirring was stopped.
[0035] Take a 1000ml reaction bottle, add 2-bromothiophene (74.5g, 0.457mol) and solvent tetrahydrofuran (150ml) under the protection of nitrogen, replace with nitrogen three times, add catalyst DPPE.NICl2 (0.48g, 0.0009mol) and 120-mesh sieve, particle size 125-250μm), temperature control 20-25°C, dropwise add the above-prepared Grignard reagent, 5h complete dropwise, GC central control. After the reaction was complete, it was quenched with 5% dilute hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com