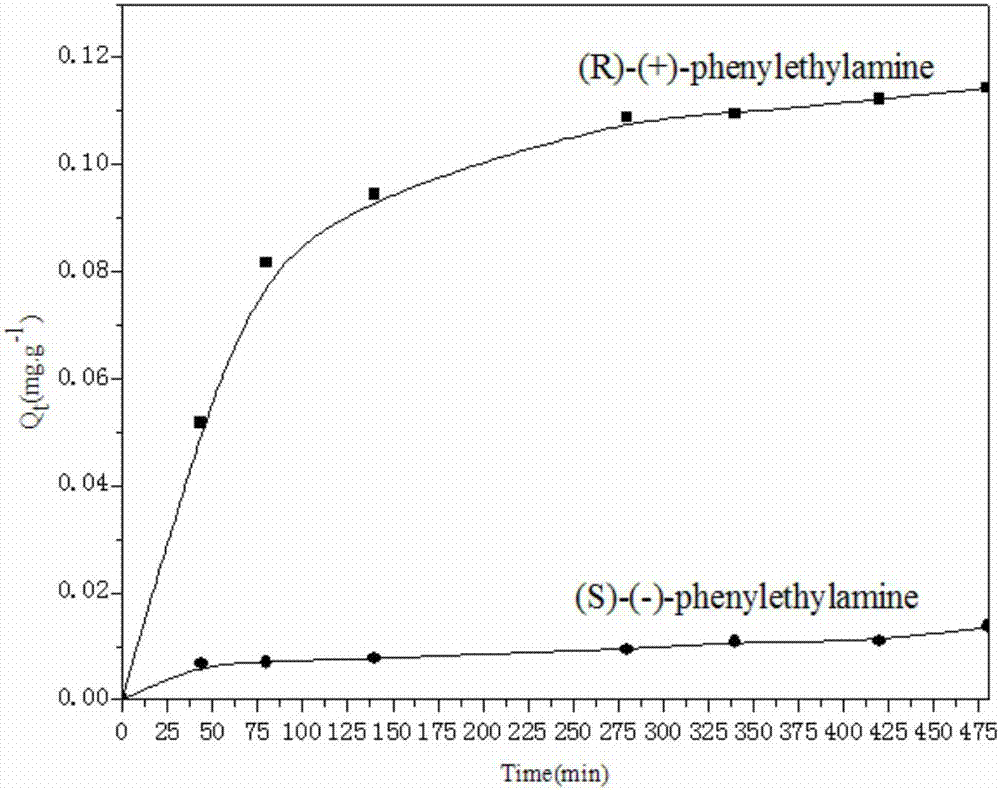
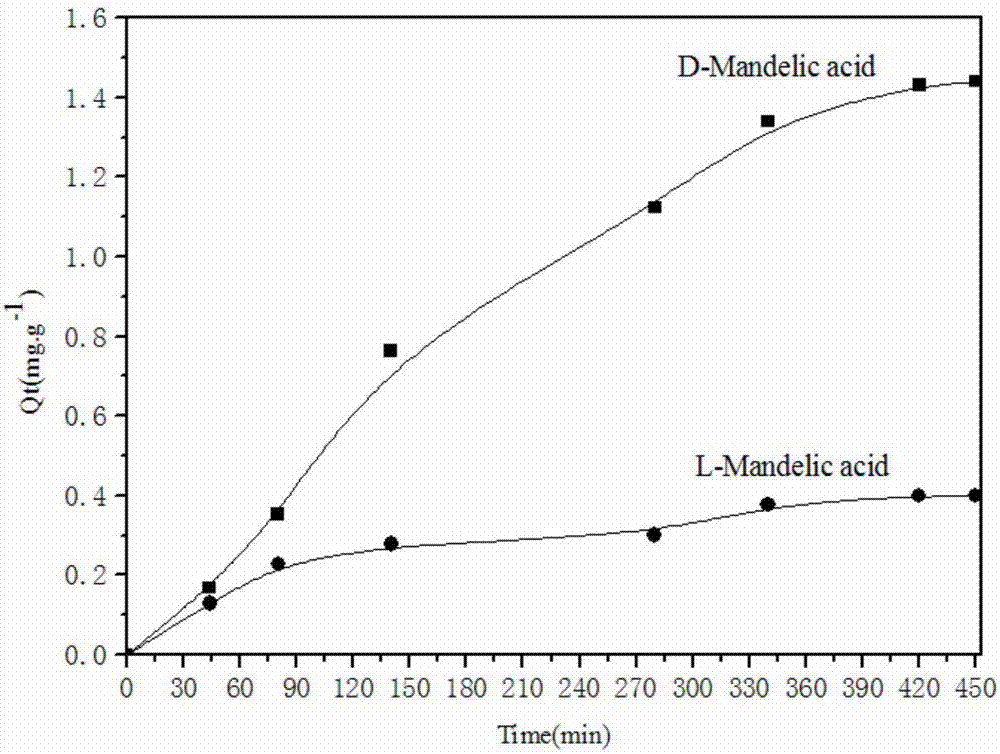
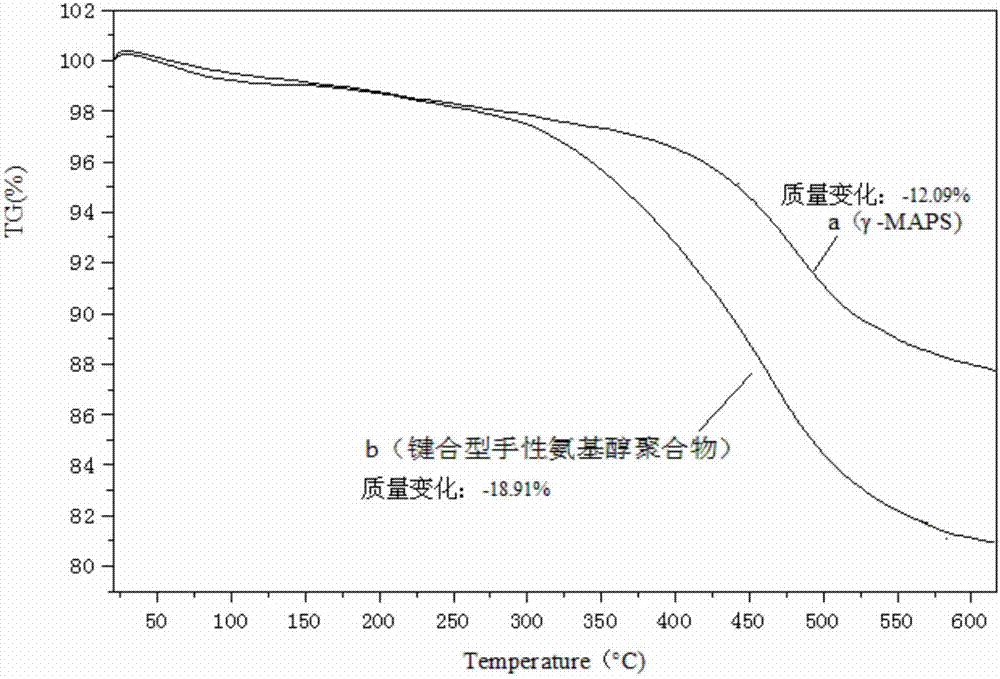
A kind of bonded chiral aminoalcohol polymer and its preparation method and application
A chiral amino alcohol and polymer technology, which is applied in the preparation of organic compounds, the preparation of carboxylic acid amides, chemical instruments and methods, etc., can solve the problems of easy loss of bonding rate of chiral selectors, and achieve strong chiral recognition ability. , to avoid loss, the effect of good physical and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of chiral monomer: Add 485mL of dry methanol into a 1000mL round bottom flask, suspend 30g (0.2287mol) L-leucine in anhydrous methanol, and dissolve excess SOC1 2 (80mL, 1.102mol) was added dropwise to the anhydrous methanol suspension, and the drop was completed within 30min, and then transferred to room temperature and stirred for 40h. The solvent was spun off, a certain amount of methanol was added and the solvent was spun dry to remove the generated hydrogen chloride and excess thionyl chloride to obtain white needle-like solid L-leucine methyl ester hydrochloride. At 30°C, add 200 mL of anhydrous tetrahydrofuran and magnesium chips (26.02 g, 1.0842 mol) into a 1000 mL three-necked flask, install a condenser tube and a dropping funnel containing 114 mL of bromobenzene and 50 mL of tetrahydrofuran, and put 2 mL of The anhydrous tetrahydrofuran solution of bromobenzene (0.06mol) is slowly dropped into the reaction system, and then a small amount of iodine ...
Embodiment 2
[0069] Preparation of chiral monomer: under ice-water bath cooling, excess SOC1 2 (0.225mol) was added dropwise to 150mL of L-leucine (0.075mol) methanol suspension, the drop was completed within 30min, and then transferred to room temperature and stirred for 24h. The solvent was spun off, a certain amount of methanol was added and the solvent was spun dry to remove the generated hydrogen chloride to obtain 13.3 g of L-leucine methyl ester hydrochloride as a white needle-like solid. At 30°C, add 50 mL of anhydrous diethyl ether and magnesium chips (0.06 mol) into a 100 mL three-necked flask, install a condenser tube and a dropping funnel, and add 2 mL of a diethyl ether solution of bromobenzene (0.06 mol) dropwise to the reaction system. Add iodine to initiate the reaction. After the color of iodine disappears, that is, after the reaction is initiated, slowly add the remaining ether solution of bromobenzene to keep the reaction stable and slightly boiling. The dripping is comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com