Synthetic method of a class of 9,9'-spirobifluorene derivatives
A technology of spirobifluorene and its derivatives, which is applied in the field of organic chemical synthesis, can solve the problems of 2-bromobiphenyl being expensive, high price, difficult industrial production, etc., and meet the needs of industrial production with low cost and reduced cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017]
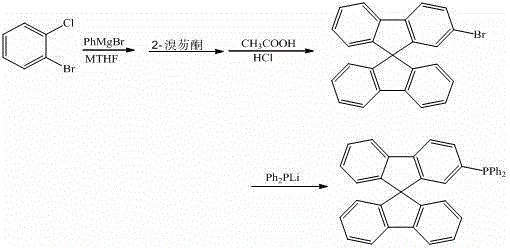
[0018] Under the protection of argon, the reaction temperature was controlled at 100°C, 10 mL of o-bromochlorobenzene (0.1 mmol) in methyl tetrahydrofuran was dropped into 5 mL of phenylmagnesium bromide (0.15 mmol) in methyl tetrahydrofuran, and the reaction was stirred for 20 h. Then the above reaction solution was dropped into 10mL of 2-bromofluorenone (0.1mmol) ether solution, refluxed for 2h, hydrolyzed, filtered, and the solid was reacted in 5mL of mixed acid (glacial acetic acid and hydrochloric acid) at 75°C for 4h, filtered, and the crude solid Using dichloromethane-n-hexane mixed solvent as eluent for column chromatography (200-300 mesh silica gel), 30.0 mg of 2-bromo-9,9'-spirobifluorene was obtained with a yield of 76.0%. The obtained 2-bromo-9,9'-spirobifluorene was reacted with lithium diphenylphosphine (0.075 mmol) in 10 mL of tetrahydrofuran solvent, and after reflux for 5 hours, 10 mL of methanol was added to the reaction solution, and the solid was...
example 2
[0024]
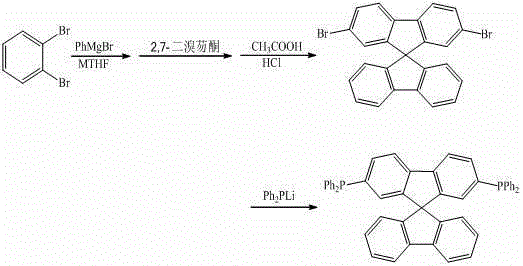
[0025] Under the protection of argon, the reaction temperature was controlled at 80°C, 10 mL of o-dibromobenzene (0.1 mmol) in methyl tetrahydrofuran was dropped into 5 mL of phenylmagnesium bromide (0.11 mmol) in methyl tetrahydrofuran, and the reaction was stirred for 12 h. Then drop the above reaction solution into 10mL of 2,7-dibromofluorenone (0.1mmol) ether solution, heat to reflux for 2h, hydrolyze, filter, react the solid in 5mL of mixed acid (glacial acetic acid and hydrochloric acid) at 75°C for 4h, and filter , the obtained solid was separated by column chromatography (200-300 mesh silica gel) using dichloromethane-n-hexane mixed solvent as eluent to obtain the product 2,7-dibromo-9,9'-spirobifluorene 36.2mg, yield 76.2% . The 2,7-dibromo-9,9'-spirobifluorene obtained above was reacted with lithium diphenylphosphine (0.15 mmol) in tetrahydrofuran solvent, and after reflux for 12 hours, methanol was added to the reaction solution, and a solid was obtained...
example 3
[0031]
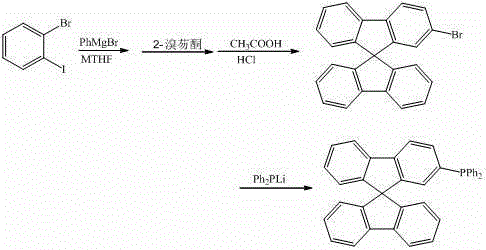
[0032] Under the protection of argon, the reaction temperature was controlled at 50°C, 10 mL of o-bromoiodobenzene (0.1 mmol) in methyl tetrahydrofuran was dropped into 5 mL of phenylmagnesium bromide (0.09 mmol) in methyl tetrahydrofuran, and the reaction was stirred for 8 h. Then the above reaction solution was dropped into 5mL of 2-bromofluorenone (0.1mol) ether solution, refluxed for 2h, hydrolyzed, filtered to obtain a solid, reacted in 10mL of mixed acid (glacial acetic acid and hydrochloric acid) at 75°C for 4h, filtered to obtain a solid for use Dichloromethane-n-hexane mixed solvent was used as eluent for column chromatography (200-300 mesh silica gel), and dried to obtain 30.9 mg of 2-bromo-9,9'-spirobifluorene, with a yield of 78.2%. The 2-bromo-9,9'-spirobifluorene obtained above was reacted with lithium diphenylphosphine (0.075 mmol) in 5 mL of tetrahydrofuran, and after reflux for 5 hours, methanol was added to obtain a solid, which was washed with wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com