Method for synthesizing AHU377 calcium salt
A technology of AHU377 and synthesis method, applied in the directions of organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems such as the unreported synthesis of AHU377 calcium salt route, etc., and achieves low comprehensive cost, mild reaction conditions, and yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
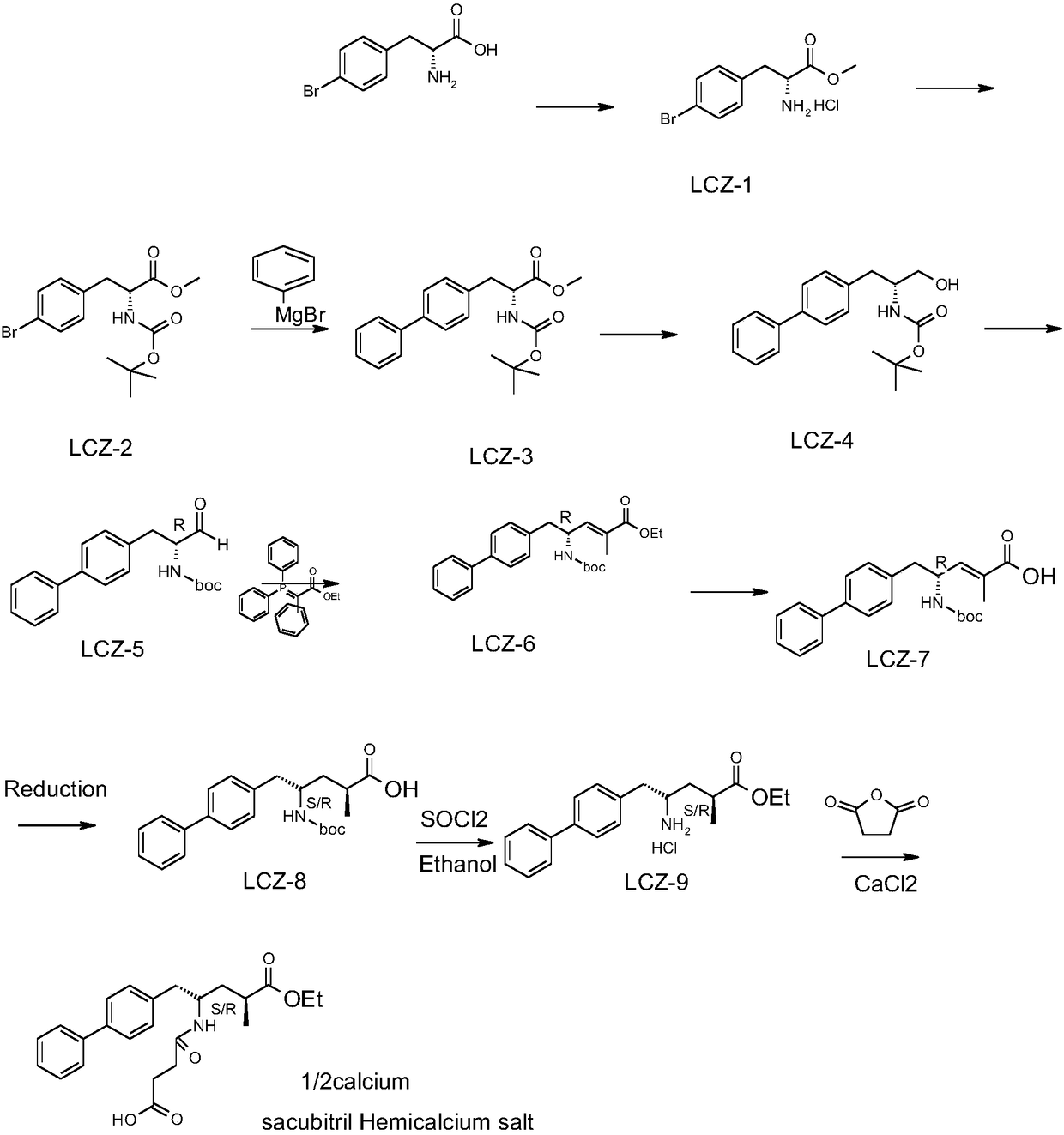
[0027] Embodiment 1 A kind of synthetic method of AHU377 calcium salt, comprises the steps:
[0028] 1) Add 500g of 4-bromo-D-phenylalanine and 2.5L of methanol into the reaction flask, add 217.7ml of thionyl chloride dropwise, after the addition is complete, raise the temperature to 65°C, keep the reaction for 1 hour, concentrate and crystallize under reduced pressure at 40°C Obtain 482g 4-bromo-D-phenylalanine methyl ester hydrochloride;
[0029] 2) Dissolve 482g of 4-bromo-D-phenylalanine methyl ester hydrochloride in 4.8L of dichloromethane, add 392.9g of BOC anhydride and 938mLkg of triethylamine, keep the reaction at 20-25°C for 3 hours, and the reaction is over Afterwards, concentrated crystallization obtains 469g Boc-4-bromo-D-phenylalanine methyl ester (80% yield);
[0030] 3) Mix 284.5g phenylmagnesium bromide and 469g Boc-4-bromo-D-phenylalanine methyl ester, -15 ~ Reaction at -20°C for 2 hours, the reaction was completed, raised to room temperature, concentrated ...
Embodiment 2
[0037] Embodiment 2 A kind of synthetic method of AHU377 calcium salt, comprises the steps:
[0038] 1) Add 250g of 4-bromo-D-phenylalanine and 1.25L of methanol into the reaction flask, add 108.85ml of thionyl chloride dropwise, after the addition is complete, raise the temperature to 65°C, keep the temperature for 1 hour, and crystallize under reduced pressure at 40°C to obtain 241g 4-bromo-D-phenylalanine methyl ester hydrochloride;
[0039] 2) Dissolve 241g of 4-bromo-D-phenylalanine methyl ester hydrochloride in 2.4L of dichloromethane, add 196.5g of BOC anhydride and 469mLkg of triethylamine, keep the reaction at 20-25°C for 3 hours, and the reaction is over Afterwards, concentrated and crystallized to obtain 234.5g Boc-4-bromo-D-phenylalanine methyl ester (80% yield);
[0040] 3) Mix 142.3g phenylmagnesium bromide and 234.5g Boc-4-bromo-D-phenylalanine methyl ester, -15 ~ -20 DEG C reacted for 2 hours, the reaction was finished, rose to room temperature, concentrated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com